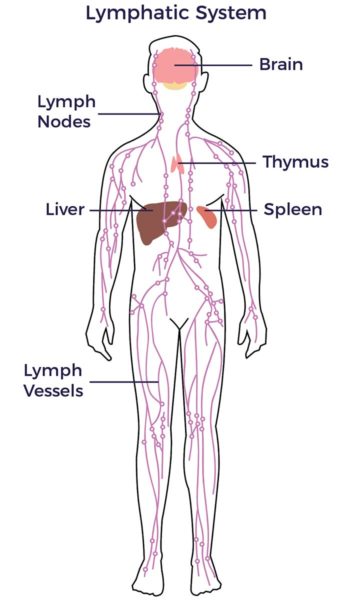
Many sources of stem cells exist, not all of them are created equal. At AMBROSE, having examined these various sources and their functions, our interest is in the adult stem cells found in adipose tissue (fat).
Adipose-derived stem and regenerative cells (ADRCs) are scientifically validated to be more accessible, abundant and potent than the stem cell populations from bone marrow and umbilical cord blood. They also have an advantaged mix of cell types to rescue, repair and regenerate damaged tissues, organs and the systems—including the vascular, immune, autonomic, and endocrine (hormones)—of our bodies. In addition, the factors of age-related declines in yield and potency that are found in the in the bone marrow are not observed in ADRCS. [1] [2] [3]
What is a Stem Cell?
The term stem cell first appeared in the scientific literature some 150 years ago in the works of the eminent German biologist Ernst Haeckel (Haeckel, 1868). Haeckel, a major supporter of Darwin’s theory of evolution, drew a number of branching trees to represent the evolution of organisms by descent from common ancestors and called these trees ‘‘Stammba ̈ ume’’ (German for family trees or ‘‘stem trees’’). In this context, Haeckel used the term ‘‘Stammzelle’’ (German for stem cell) to describe these cells as the most basic cells in our body and from which all other cells are developed.
Embryonic Stem Cells
Embryonic stem cells (ESCs)—of which there are between 50 and 150—are developmental cells, meaning they develop into all of the cell lines of our body and those cells replicate. They are referred to as “totipotent stem cells,” being the only cells capable of giving rise to all cell types necessary to develop an embryo into a fully formed body.
ESCs can develop into each of the more than 200 cell types of the adult body when given sufficient and necessary stimulation for a specific cell type, however ethical and medical concerns surround their use. Due to this they have been the subject of only a small number of human trials and are illegal to use in nearly all developed countries including the US.
Adult Stem Cells
- Hematopoietic Stem Cells – Blood cell forming stem cells found in the bone marrow and umbilical cord tissue and blood
- Mesenchymal stem cells (MSCs) – Reparative stem cells found in every tissue of the body, most abundantly in our fat.
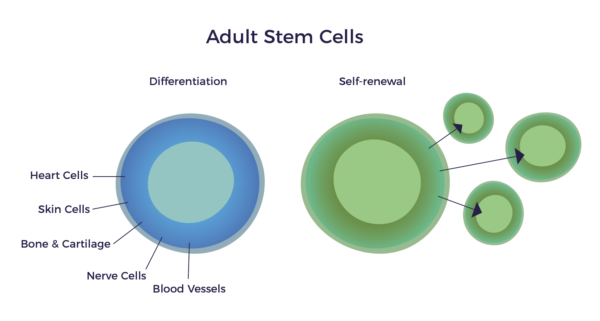
The term “adult stem cell” is cause for some confusion, as these are the cells we are born with. Adults and infants alike possess the same “adult” stem cells. Thus, a more accurate and descriptive name for these cells might be “repair cells,” for this is their function in a fully developed body. Adult stem cells do not grow a new heart, brain, or bones—they are purposed to keep what we have in good repair.
The history of adult stem cell research began in the 1950s, when researchers discovered that the bone marrow contains at least two kinds of stem cells. The first population, called hematopoietic stem cells (HSCs), forms all the types of blood cells in the body. HSCs make up approximately 99.9% of the stem cells in bone marrow and, as they are blood forming stem cells, they are used to treat blood cancers. Umbilical cord blood stem cells are also virtually all HSCs and have been approved for use to treat blood cancers as well.[4] There are stem cells in the umbilical cord tissue but as they are small in number per gram it is necessary to grow them out in a dish (culture).
The second population found in bone marrow is the mesenchymal stem cell (MSC).
The origin of the concept of a “mesenchymal” stem cell goes back to the pioneering experiments of Tavassoli and Crosby in the 1960s.[5] In 1991, Prof. Arnold Caplan from Case-Western University named the MSC. [6] According to a Medline search, there are now over 49,000 published papers on MSCs and they have been used in over 700 clinical trials. In 2016, Dr. Caplan, in MSCs—The Sentinel and Safe-Guards of Injury[7] proposed renaming mesenchymal stem cells to “medicinal signaling cells” due to their function of repairing through cell-to-cell communication or the “paracrine effect.”
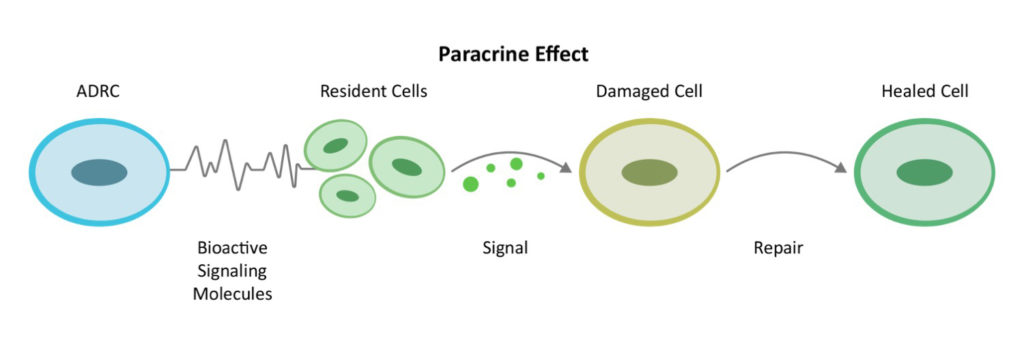
 MSCs are non-blood cell-making stem cells found in every tissue of our bodies. They make up a rare proportion of the population in the bone marrow and can differentiate into bone, cartilage and fat cells. The early adult stem cell research focused on bone marrow source but one key problem arose: The quantity and potency of bone marrow stem cells decline with age as well as in the presence of chronic diseases.
MSCs are non-blood cell-making stem cells found in every tissue of our bodies. They make up a rare proportion of the population in the bone marrow and can differentiate into bone, cartilage and fat cells. The early adult stem cell research focused on bone marrow source but one key problem arose: The quantity and potency of bone marrow stem cells decline with age as well as in the presence of chronic diseases.
Advantages of Adipose Tissue
There is more to fat than meets the eye. Whereas many researchers focus solely on the MSCs found in bone marrow, fat, umbilical cord, placenta, dental pulp and so on, in fat they are not unlike a lead singer in a band whose music is enriched and enhanced by backup singers and the other musicians. There are many other cell types in fat that work together, and we call these “regenerative cells.”
Adipose tissue functions as a protected reservoir of stem and regenerative cells throughout one’s life. The studies using bone marrow mononuclear cells (BMNCs) for bone marrow found a steep drop-off in efficacy after the age of 62.[8]
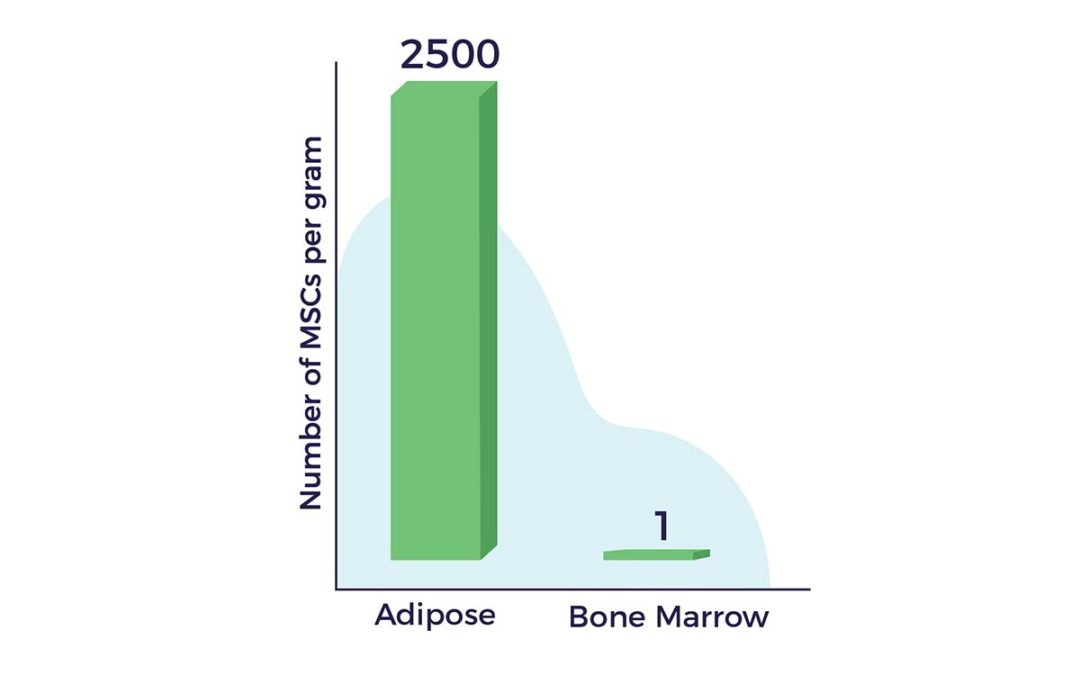
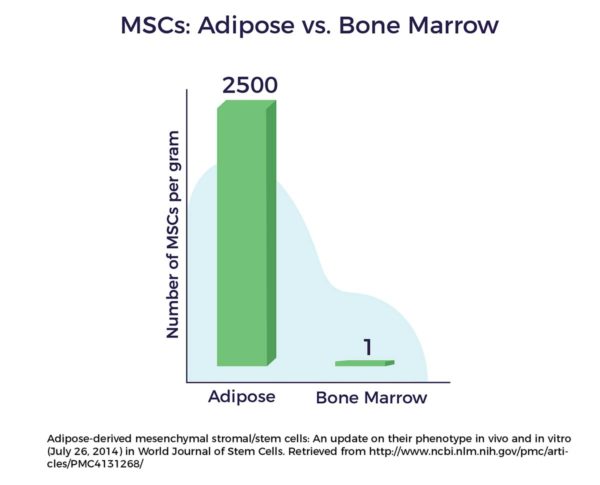
Another advantage of fat as a cell source lies in the fact that there are up to 2,500 times more MSCs in a gram of adipose tissue vs a gram of bone marrow. [9]
Fat-derived regenerative cells were first discovered in 1964 by Martin Rodbell who, using an enzyme and a centrifuge, successfully liberated a mixed population of cells from the adipose tissue of a mouse. Research on adult stem cells took a quantum leap forward in 2001, when Patricia Zuk PhD, Dr. Marc Hedrick and others working in the labs of UCLA, published a paper in Tissue Engineering discussing their discovery that mesenchymal stem cells (MSCs) reside alongside other regenerative cells in our fat.[10] With that a new era in medicine had begun.
Different Cells, Different Jobs
Adult stem cells from different cells sources work for different indications including certain blood cancers for bone marrow and umbilical cord stem cells. Bone marrow stem cells have been shown to be most effective in orthopedic and cardiac cell therapy in middle-aged or younger patients due to the declining number and potency as we age. Conversely, ADRCs are accessible, abundant and potent later in life and hence hold the most promise for age-related degenerative diseases.