Spinal Cord Injury Cell Therapy Hypothesis
Background: In December 2018, AMBROSE Cell Therapy pioneered a novel protocol for Jeff, a patient living with a spinal cord injury. Jeff’s 14-month patient-reported outcome chronicled significant improvements in symptoms, function, and quality of life.
In December 2017, Jeff’s spine surgery gone wrong resulted in an SCI and paraplegia. His extended hospitalization led to an abscessed colon requiring an ileostomy. Compounding matters, he lived with T2 diabetes.
Jeff complained of neck and back pain, neuropathy, scar pain from the ileostomy, claw toes, spasticity, poor balance, diabetes T2, shoulder immobility, and erectile dysfunction (ED). He took seven meds to manage his symptoms, including opioids.
Our in-depth review of published SCI stem cell therapy studies yielded positive results. Thus, AMBROSE curated the literature and developed a personalized protocol for Jeff.
The treatment strategy was unconventional. Rather than only targeting the spinal cord lesion, the AMBROSE medical team addressed Jeff’s array of complaints in a single procedure using a Master Protocol.
In essence, AMBROSE designed one overarching protocol to treat multiple issues in a single treatment including the injury site, using adipose-derived regenerative cells (ADRCs). Our treatment team then personalizes it for each patient.
Importantly, our previous group’s prior successes using a Master Protocol with over 300 patients living with diverse chronic conditions supported our hypothesis. (Okyanos Cell Therapy, Bahamas 2014- 2017).
In addition:
- A subsequent meta-analysis, Transplantation of mesenchymal stem cells for spinal cord injury: a systematic review and network meta‑analysis, validated the safety and effectiveness of MSC-based treatments for SCI. [1]
- Researchers had published successful case reports using injections of micronized adipose tissue to address SCI-related musculoskeletal conditions, scar pain, and neuropathy. [2] [3]
- Published trials for other chronic conditions informed.[4] [5]
- Urology KOLs advised on a sacral-plexus injection sub-protocol to target bladder and bowel dysfunction.
Hypothesis
A wide gap exists between the known pathophysiology of spinal cord injury and the standard of care being relied upon to treat it. Specifically, the damage to the cord incites multisystem dysregulation, including immune, endothelial, metabolic, mitochondrial, and autonomic dysfunction. [6] [7] [8]
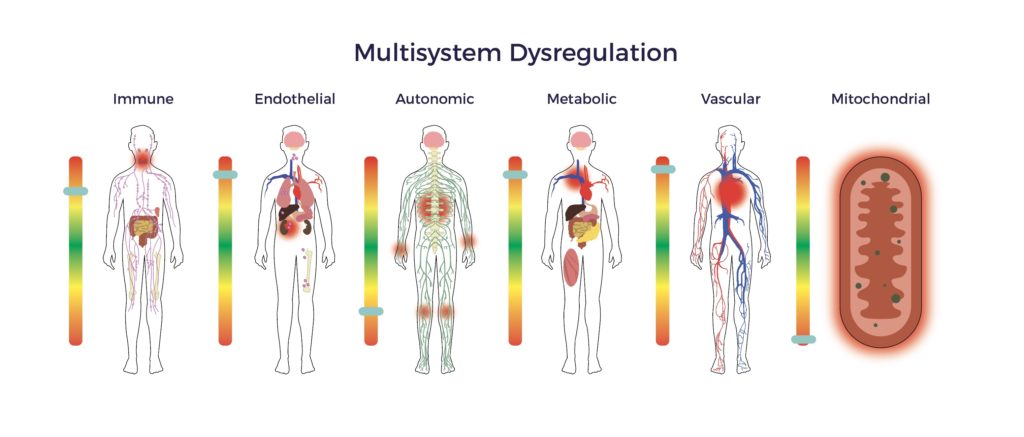
Hence, patients suffer from an array of secondary complications.
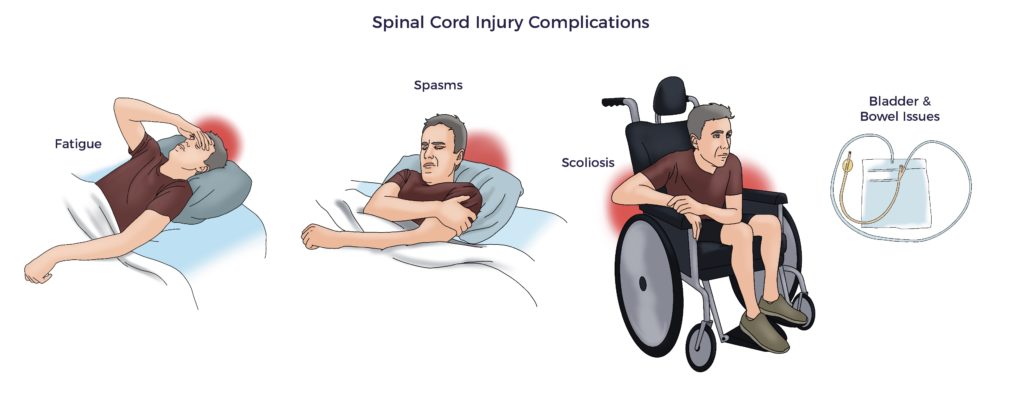
Further, the overwhelming insult suppresses spinal cord regeneration, limiting functional recovery. Thus, the expected window of improvement for SCI patients is ~12 months.
As an example, Jeff’s symptoms included 24/7 scar pain, neuropathy, bilateral spasticity, bowel and bladder issues, ED, and impaired balance. At 11 months post-injury, Jeff and his physiatrist recognized his progress had leveled off.
Yet, drug discovery focuses on a single mechanism of action to repair the injury site or suppress a symptom. Put differently, the pharma model of one drug, gene, or cell type to treat a disease has made little progress in improving the prognosis for patients living with spinal cord injuries. This approach neglects a deep body of literature documenting the multiple factors and resulting comorbidities that combine to handicap patients like Jeff.
Multiple Mechanisms of ADRCs
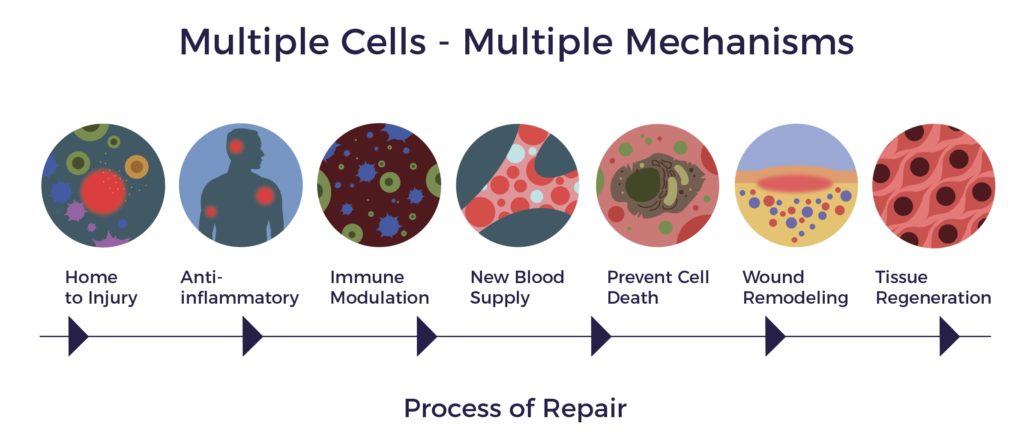
In contrast, ADRCs have multiple mechanisms of action (MOAs) targeting all the factors involved in a chronic condition, including multisystem dysregulation. [9]
ADRCs home to sites of inflammation and secrete hundreds of bioactive molecules that are anti-inflammatory, immuno-modulatory, angiogenic, anti-apoptotic, and wound healing.
ADRCs work through cell-to-cell communication or the paracrine effect.
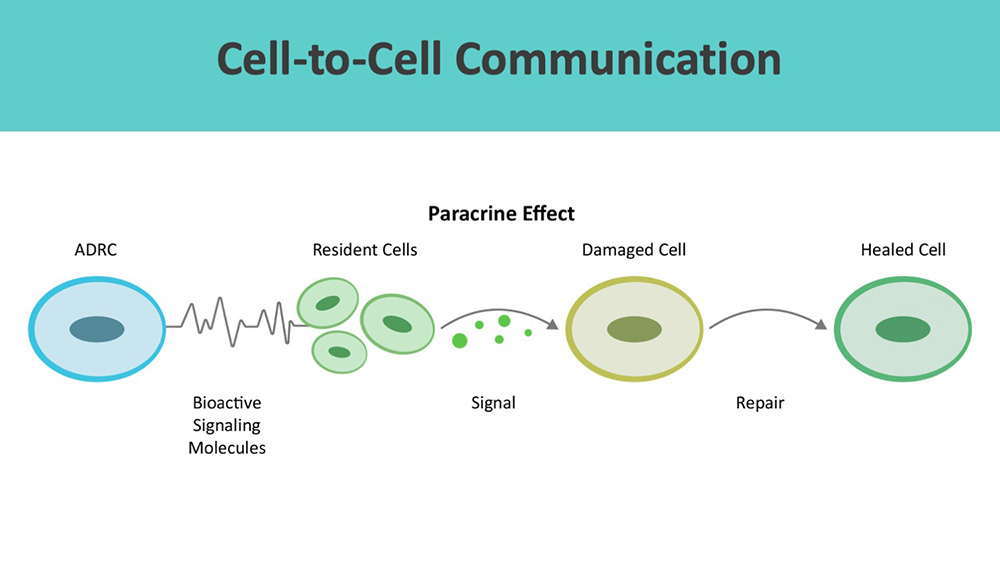
Beyond wound healing mechanisms, ADRCs restore cellular, systemic, and tissue equilibrium (multisystem homeostasis). [10] [11]
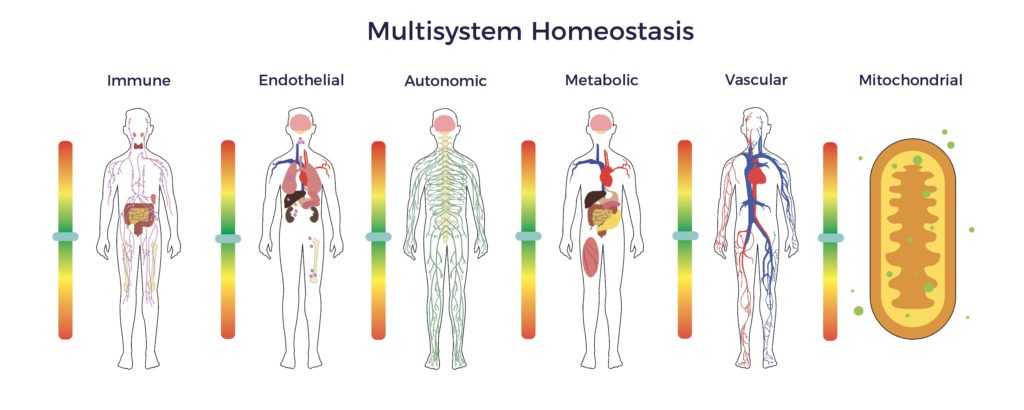
In Jeff’s recovery, the restoration of multisystem homeostasis included improved balance, pain scores, bladder, and bowel function, as well as a significant reduction in spasticity. The net result was an elimination of all drugs, engaging in hardcore gym workouts, a return to racing, learning to fly helicopters and scuba diving, plus playing golf three times a week. Though not cured or asymptomatic, Jeff is real-world evidence that ADRCs extended Jeff’s recovery window.

In sum, our preliminary evidence indicates that ADRCs can extend or restart the neurologic window of recovery by restoring multisystem homeostasis.
Why Adipose Tissue?
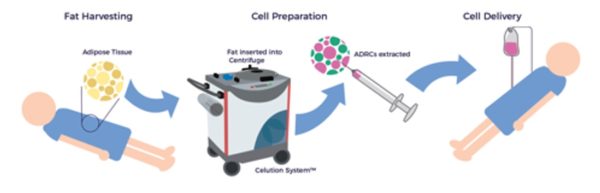
Unlike other tissue sources of adult stem cells, e.g., bone marrow, ADRCs remain accessible, abundant, and potent throughout one’s life. [12] Following liposuction, the Celution™ Cell Processing System (Lorem Cytori, Inc.) centrifuges the lipoaspirate and prepares the ADRCs.
Why ADRCs
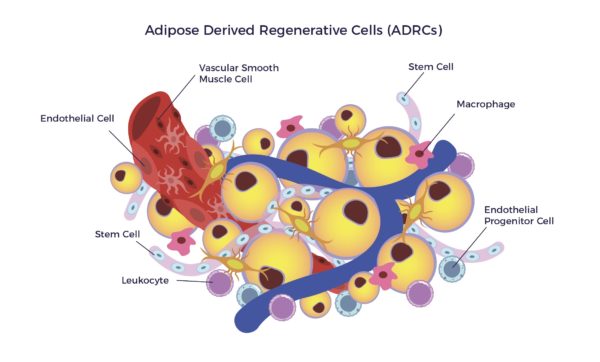
Adipose-Derived Regenerative Cells (ADRCs) are the designation for a clinical-grade preparation of stromal vascular fraction (SVF), a heterogeneous population of cells residing on the outer lining of the capillaries in adipose tissue. The cell mix includes MSCs, endothelial cells (ECs), endothelial progenitor cells (EPCs), fibroblasts, T-regulatory cells (Tregs), macrophages, and other immune cells (leukocytes).[13]
ADRCs secrete significantly higher amounts of trophic factors compared to Adipose-Derived MSCs (ADSCs).[14] Therefore, the ADRC secretome amplifies the MOAs of ADSCs, including but not limited to:
- Anti-inflammation and Immuno-modulation,
- Anti-apoptosis,
- Angiogenesis,
- Support of growth,
- Differentiation of local stem cells and progenitor cells,
- Anti-scarring, and
- Chemoattraction.
Human studies show ADRCs:
- Release factors that down-regulate inflammatory-autoimmune markers, including but not limited to TNF-A, TH17, IL6, and IL2, as well as upregulate IL10.
- Promote the switch from inflammatory macrophages (M1s) to anti-inflammatory macrophages (M2s) via Prostaglandin E2 (PGE2) and the MSCs in the mix.
- Reduce Endothelin-1 (ET-1), a known constrictor of blood vessels, implicated in spinal cord-blood-barrier disruption after the injury.
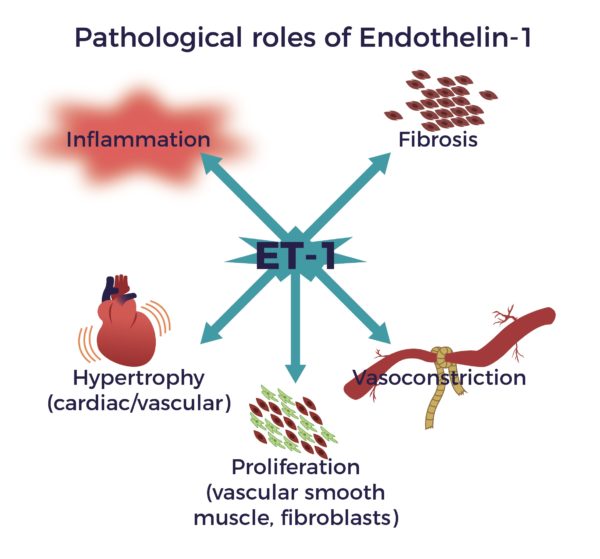
- Assist in the growth and stabilization of new blood vessels by secreting placental Growth Factor (PGF), Stromal-Derived Factor-1 (SDF-1), and Vascular Endothelial Growth Factor (VEGF).
Notably, a close relationship exists between revascularization and improved functional outcomes after SCI. First, a well-vascularized lesion provides a permissive microenvironment for local tissue survival and nerve regeneration. Improved capillary blood flow, angiogenesis, and B-SC-B integrity facilitate functional recovery.[15] [16]
Neurotrophic factors
ADRCs deliver neurotrophic factors (NTFs) to the CNS, PNS, and ANS. Just as fertilizers keep plants healthy and growing, NTFs stimulate the development of new brain cells, brain cell connections, and nerves.
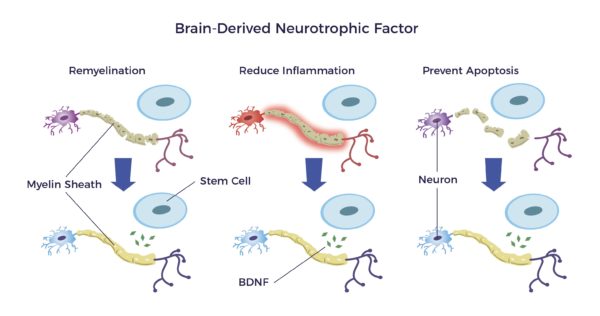
One such trophic factor is the brain-derived neurotrophic factor or BDNF.
BDNF:
- Repairs the myelin sheathing surrounding the nerves
- Reduces inflammation and,
- Prevents apoptosis that results from an injury or disease. [17] [18] [19] [20] [21]
A recent discovery of neuro-immune cells in ADRCs connects their role in enhancing neuroplasticity.[22] [23]
Safety
Since 2007, no ADRC-related adverse events have been reported.[24]
[1] Chen et al Transplantation of mesenchymal stem cells for spinal cord injury: a systematic review and network meta‑analysis J Transl Med (2021) 19:178
[2] Cherian C et al. Autologous, micro-fragmented adipose tissue as a treatment for chronic shoulder pain in a wheelchair using individual with spinal cord injury: a case report Spinal Cord Series and Cases (2019) 5:46
[3] Huang S-H, Wu S-H, Lee S-S, Chang K-P, Chai C-Y, Yeh J-L, et al. (2015) Fat Grafting in Burn Scar Alleviates Neuropathic Pain via Anti- Inflammation Effect in Scar and Spinal Cord. PLoS ONE 10(9): e0137563
[4] A Nguyen, A et al Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature Journal of Plastic, Reconstructive & Aesthetic Surgery (2016) 69, 170e179
[5] Guo et al Stromal vascular fraction: A regenerative reality? Part 2: Current concepts and review of the literature Journal of Plastic, Reconstructive & Aesthetic Surgery (2016) 69, 180e188
[6] Sun et al. Multiple organ dysfunction and systemic inflammation after spinal cord injury: a complex relationship Journal of Neuroinflammation (2016) 13:260
[7] B. Perrouin-Verbe, C. Lefevre, P. Kieny, R. Gross, B. Reiss, M. Le Fort, Spinal cord injury: A multisystem physiological impairment/dysfunction, Revue Neurologique, Volume 177, Issue 5, 2021, Pages 594 605,
[8] Marion et al. Previously Identified Common Post-Injury Adverse Events in Traumatic Spinal Cord Injury—Validation of Existing Literature and Relation to Selected Potentially Modifiable Comorbidities: A Prospective Canadian Cohort Study JOURNAL OF NEUROTRAUMA 34:2883–2891 (October 15, 2017)
[9] Caplan A I Mesenchymal Stem Cells: Time to Change the Name! STEM CELLS TRANSLATIONALMEDICINE 2017; 6:1445–1451
[10] Visoso F. J. et al Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities Int. J. Mol. Sci. 2019, 20, 3738
[11] Morris, M.E. et al. (2015), Systemically Delivered Adipose Stromal Vascular Fraction Cells Disseminate to Peripheral Artery Walls and Reduce Vasomotor Tone Through a CD11b+ Cell-Dependent Mechanism. STEM CELLS Translational Medicine, 4: 369-380.
[12] Perin EC and Willerson JT Buying New Soul J Am Coll Cardiol. 2012;60(21):2250-2251
[13]S Kesten and JK Fraser Autologous Adipose Derived Regenerative Cells: A Platform for Therapeutic Applications Advanced Wound Healing Surgical Technology International XXIX
[14] Hirosi Y et al. Comparison of trophic factors secreted from human adipose-derived stromal vascular fraction with those from adipose-derived stromal/ stem cells in the same individuals
[15]Yao C, Cao X and Yu B (2021) Revascularization After Traumatic Spinal Cord Injury. Front. Physiol. 12:631500.
[16] Numan MT et al. Autologous Adipose Stem Cell Therapy for Autonomic Nervous System Dysfunction in Two Young Patients. Stem Cells and Development 2017 26:6, 391-393
[17] Razavi, Shahnaz et al. “Neurotrophic Factors and Their Effects in the Treatment of Multiple Sclerosis.” Advanced Biomedical Research 4 (2015): 53. PMC. Web. 28 Sept. 2018.
[18] J. K. Huang et al Myelin Regeneration in Multiple Sclerosis: Targeting. Endogenous Stem Cells., The American Society for Experimental NeuroTherapeutics, Inc. 2011
[19] T Lopatina et al. (2011) Adipose-Derived Stem Cells Stimulate Regeneration of Peripheral Nerves: BDNF Secreted by These Cells Promotes Nerve Healing and Axon Growth De Novo. PLoS ONE 6(3): e178991
[20] S. Seigo et al, Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration, Journal of Orthopaedic Science, Volume 18, Issue 1,2013, Pages 145-151
[21] Xu et al Brain-derived neurotrophic factor reduces inflammation and hippocampal apoptosis in experimental Streptococcus pneumoniae meningitis Journal of Neuroinflammation (2017) 14:156
[22] O’Reilly ML and Tom VJ (2020) Neuroimmune System as a Driving Force for Plasticity Following CNS Injury. Front. Cell. Neurosci. 14:187.
[23] Blaszkiewicz, M., Wood, E., Koizar, S. et al. The involvement of neuroimmune cells in adipose innervation. Mol Med 26, 126 (2020)
[24] Lorem Cytori, Inc Unpublished internal data
