What Do Umbilical Cord Stem Cells and Tide Pods Have in Common
 “People often ask if stem cells work. Of course, they work. We are all walking stem cell products – the sperm, and the egg,” the revered James Willerson, MD, Ph.D. said in a talk in 2011.[1]
“People often ask if stem cells work. Of course, they work. We are all walking stem cell products – the sperm, and the egg,” the revered James Willerson, MD, Ph.D. said in a talk in 2011.[1]
The Tide Pod Teenage Eating Fad, Gronk, and Reverse Psychology
What do dentists, chiropractors, anti-aging doctors, the Four Seasons Maui Spa, and the Tide Pods Eating Fad Have in Common?

In 2018, the Teenagers Eating Tide Pods Fad resulted in 10 deaths and 37 reported poison cases.
Proctor and Gamble, Tide’s manufacturer, went on the offensive to protect kids from poisoning themselves.
They even hired NFL superstar Rob Gronkowski to come from behind in the clutch as he had done to win four Super Bowls with Tom Brady. However, Gronk’s YouTube Tide Pod admonishment resulted in more teens taking the challenge.
Contamination, the FDA, and Reverse Psychology
On December 5, 2019, the FDA warned Liveyon Labs, Inc. that they were selling unapproved, contaminated umbilical cord stem cell products. At least 300 patients had reported bacterial infections tied to Liveyon’s umbilical cord stem cell products.
One day later, the agency issued a press release informing manufacturers, providers, and the public of Liveyon’s issues and the broader scope of their concerns. The other recipients were RichSource Stem Cells, Inc., Chara Biologics, Inc., and R3 Stem Cell, LLC. In total, FDA issued 350 warning letters to manufacturers, healthcare providers, and clinics.
The result: Increasingly, chiropractors, dentists, naturopaths, integrative doctors, infusion clinics, orthopedists, and so on treat patients with unapproved, potentially contaminated, dead stem cells.
Who Killed the Stem Cells Sadly, as a chiropractor’s patient with long-Covid and congestive heart failure related, “I tried umbilical cord stem cells. They didn’t work.” There were no live stem cells in the product with which he was treated or other commercially available perinatal vials, studies revealed:
Sadly, as a chiropractor’s patient with long-Covid and congestive heart failure related, “I tried umbilical cord stem cells. They didn’t work.” There were no live stem cells in the product with which he was treated or other commercially available perinatal vials, studies revealed:
- Cell viability in the cord blood product was less than reported by the manufacturer, the cells were primarily leukocytes, no stem cells were present…”[2]
- The aggressive marketing approach currently used by practitioners and clinics regarding various birth tissue products as safe and effective “stem cell therapy” is not supported by the existing scientific literature. [3]
- CFU-Fs, often referred to as stem cells, were not found within any of the commercial UC (umbilical cord) allograft products analyzed, and clinicians should remain wary of marketing claims stating otherwise. [4]
- Amniotic fluid has been proposed as an allogenic means for introducing MSCs. This study was unable to confirm that commercial AFPs (amniotic fluid products) contain MSCs. [5]
Risk Appetite
But how does the Tide Pod eating fad relate to the umbilical cord stem cell fad? The Tide Pod teenagers and Umbilical Cord Stem Cell (UBSC) patients share an appetite for risk:
- The more Proctor and Gamble warned kids of the danger of eating Tide Pods, the faster the fad grows.
- The more FDA warns providers and patients of perinatal stem cells and their exosomes’ risks, the more the fad is becoming a frenzy too.
A 2016 study identified 351 businesses promoting stem cell treatments. Five years later, the same author, estimated more than four times as many businesses (1,480) sell stem cell treatments, approximately half of which (781) promote umbilical, amniotic, or exosome treatments. [6]
More on risk-taking later. First, what are “perinatal stem cells”?
Perinatal Stem Cells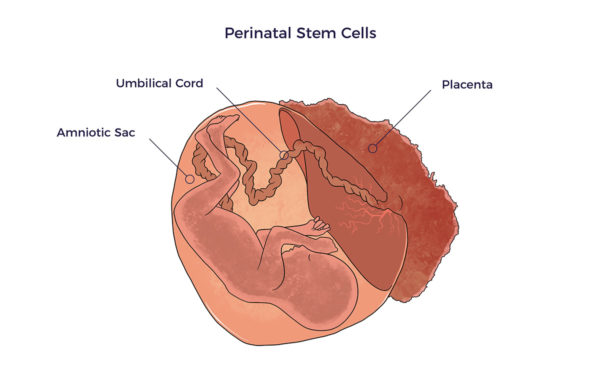 Perinatal refers to just before or shortly after birth. Thus, perinatal stem cells come from birth tissues or fluids, i.e., umbilical cord blood and tissue, placental blood and tissue, and amniotic tissue and fluid.
Perinatal refers to just before or shortly after birth. Thus, perinatal stem cells come from birth tissues or fluids, i.e., umbilical cord blood and tissue, placental blood and tissue, and amniotic tissue and fluid.
That sounds good on the surface, but as explained in The Cell Source Debate, mesenchymal stem cells (MSCs) are rare in birth tissues and fluid. There is more to that part of the story to follow.
Exosomes Cells release tiny sacs or vesicles called exosomes. Like carrier pigeons, exosomes carry messages to nearby cells.
Cells release tiny sacs or vesicles called exosomes. Like carrier pigeons, exosomes carry messages to nearby cells.
All cells with DNA – cancer, tissue, immune, blood cells, etc. secrete exosomes.
Mesenchymal stem cells (MSCs) release them too. Despite no reported side-by-side in-human studies, this mechanism has led some researchers and amateur stemcellologists to believe they are more effective than MSCs or ADRCs.
And where a therapeutic dose of MSCs might be 40 -100 million cells, exosome manufacturers claim their vials contain hundreds of millions. A South Florida “stem cell physician” touts one billion exosome vials – without evidence that more is better, much less sterile or safe.
Lab techs must multiply the MSCs or exosomes in culture to obtain a relevant dose. However, maintaining current Good Manufacturing Practices (cGMP) requires adherence to rigorous standards.
Whodunnit?

Wondery’s ten-episode whodunnit, Bad Batch, illustrates the significant safety risks Liveyon’s slick marketing and non-sterile lab practices delivered to patients. In sum, over 200 patients reported severe adverse events.
Additionally, the CDC published its lab findings in the Journal of the American Medical Association (JAMA) in 2021.
Key Points
Question Were infections in patients who received umbilical cord blood products marketed as stem cell treatment associated with product contamination?
Findings In this case series, 20 patients in 8 states, developed bacterial infections after receiving unapproved products marketed as treatment for conditions including chronic pain and degenerative joint conditions. This national investigation found widespread bacterial contamination of undistributed and distributed products from multiple donors, with whole-genome sequencing indicating a common source.
Meaning The findings from this outbreak underscore that unapproved and unproven stem cell products can expose patients to serious risks without clear benefit, including the possibility of product contamination.
Of unopened, undistributed products sampled for testing, at least 1 of 16 bacterial species contaminated 65% (22 of 34 vials). [7]
Liveyon reincarnations
Yet the warnings failed to stem the umbilical cord stem cell (UBSC) tide nor remove dirty cells:
- Utah Cord Bank May 2021 Warning Letter
- Vitti Health July 2022 Warning Letter
- Invitrix Nov 2022 Warning Letter
From an angle not addressed by the FDA, a global review of scientific literature across forty studies revealed that “forever chemicals” from toxic plastics were present in 30,000 umbilical cord blood samples.
Further, citing patient reports of adverse events from a Nebraska exosome clinic, the FDA issued a safety warning to the public.
What the hell is reactive arthritis after a “stem cell” injection?
Promoters of perinatal “stem cell” products claim there is no risk of immune rejection. In other words, they say umbilical cord Wharton’s Jelly does not require human leucocyte antigen (HLA) matching to pair patients and donors, as is done for blood or marrow transplants.
However, a mismatched case report contradicts their false claim.
“A 36-year-old man was injected with Wharton’s jelly for low back pain and within 24 hours developed fevers, chills, polyarthritis, and enthesitis. (Enthesitis is inflammation of the ‘enthesis”, which is where a tendon or ligament attaches to a bone.) Infectious disease work-up was negative. Inflammatory markers were elevated and his HLA-B27 antigen was positive. Initial treatment included methylprednisolone and sulfasalazine. This case highlights the unknown dangers of these allogenic injections and physicians should remain cautious about their use until further study and regulation can ensure patient safety.”[8]
An open penitentiary?
If these products are not FDA-approved and the labs do not comply with cGMP, how do companies get away with selling them? The manufacturers claim their products are for research purposes or ignore FDA regulations because they could care less.
- Invitrix and Vitti Labs use disclaimers to stay one step ahead of the law.
- Organicell hides behind the veil of several planned or ongoing phase 1 clinical trials.
Everybody speeds A doctor wanting to participate in the stem cell wild, wild west asked a lawyer specializing in stem cell regulatory affairs:
A doctor wanting to participate in the stem cell wild, wild west asked a lawyer specializing in stem cell regulatory affairs:
Doctor: If I need regulatory approval to treat patients with stem cells, how come so many doctors do it anyway?
Lawyer: “Do you speed?”
Doctor: “All the time.”
Lawyer: “Is it illegal?”
Doctor: “I don’t care.”
Stories like Jury Convicts State Lawmaker of COVID-19 Fraud Scheme at Springfield Health Care Charity and Man falsely claiming to use “Stem Cell Therapy” convicted and sentenced to 202 years don’t seem to make a difference.
Stem Cells and exosomes for the healthy, wealthy
NextHealth at the Four Seasons Maui Resort mixes up the mania, offering Vitti Labs’ potentially contaminated umbilical cord “stem cells” and Organicell’s unapproved exosomes in their spa. They offer Stem Cells + Exosomes + Longevity IV Therapy for $16,000, a savings of $2,299.
 Contrary to the FDA’s warning letters, a NextHealth patient coordinator insisted that Vitti’s and Organicell’s products are FDA-approved and that the manufacturers certify all lots are contaminant-free. Where did she get that idea?
Contrary to the FDA’s warning letters, a NextHealth patient coordinator insisted that Vitti’s and Organicell’s products are FDA-approved and that the manufacturers certify all lots are contaminant-free. Where did she get that idea?
From another angle, just because something is illegal doesn’t mean clever marketers partnering with iconic brands like the Four Seasons have consulted a lawyer specializing in FDA compliance. Or have read the literature cited herein.
Stem Cell Distributor expands dental practices At the other end of the spectrum, New Life, an Invitrix and Organicell distributor, “works closely with clinicians and practitioners to enhance their care offerings…and expand their practices with “natural biologics (emphasis added).” But their research products page and disclaimer tell their customers – dentists, chiropractors, nurses, and doctors – their products are not FDA-approved.
At the other end of the spectrum, New Life, an Invitrix and Organicell distributor, “works closely with clinicians and practitioners to enhance their care offerings…and expand their practices with “natural biologics (emphasis added).” But their research products page and disclaimer tell their customers – dentists, chiropractors, nurses, and doctors – their products are not FDA-approved.
Do Stem Cells Work? “Of course, they work. We are all walking stem cell products – the sperm, and the egg.” James Willerson, MD, Ph.D. Dr. Willerson went on to publish Buying New Soul (2012). Here he hypothesized that adipose tissue was the best source of adult stem cells.
“Of course, they work. We are all walking stem cell products – the sperm, and the egg.” James Willerson, MD, Ph.D. Dr. Willerson went on to publish Buying New Soul (2012). Here he hypothesized that adipose tissue was the best source of adult stem cells.
A growing body of literature supports Willerson’s supposition: A PubMed Central Search returned more than 100,000 publications that discuss adipose-derived stem cells. And over 35 published in-human Celution System studies validate the safety and effectiveness of clinical-grade ADRCs.
In contrast, experimenting on patients in dental offices or at ritzy hotels with potentially contaminated “natural biologics” that contain dead stem cells is a risky pod for patients to swallow.
[2] Fortier LA, Cercone M, Keller LE, Delco ML, Becktell L, Wells KV. Amnion and Umbilical Cord–Derived Products in Sports Medicine: From Basic Science to Clinical Application. The American Journal of Sports Medicine. 2021;49(7):1954-1961
[3]https://legislature.vermont.gov/Documents/2020/WorkGroups/Senate%20Health%20and%20Welfare/Bills/S.252/Written%20Testimony/S.252~Jonathan%20Fenton~Consensus%20Statement%20on%20Aggressive%20Marketing%20of%20Birth%20Tissues%20as%20Stem%20Cell%20Therapies~2-28-2020.pdf
[4] Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison with Autologous Orthobiologics. Am J Sports Med. 2021 Oct;49(12):3404-3413.
[5] Panero AJ, Hirahara AM, Andersen WJ, Rothenberg J, Fierro F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine. 2019;47(5):1230-1235.
[6] Turner L The American stem cell sell in 2021: U.S. businesses selling unlicensed and unproven stem cell interventions Cell Stem Cell 28, November 4, 2021
[7] Hartnett KP, Powell KM, Rankin D, et al. Investigation of Bacterial Infections Among Patients Treated with Umbilical Cord Blood–Derived Products Marketed as Stem Cell Therapies. JAMA Netw Open.2021;4(10): e2128615.
[8] Madhoun et al. Induction of HLA-B27–Associated Reactive Arthritis After a Wharton’s Jelly “Stem Cell” Injection Am J Phys Med Rehabil 2020;99:e142–e145






