A Golden Era of Cell-Assisted Neurodevelopment
Adipose Tissue – Fat’s Neurodevelopmental Potential
Here, we explore why an intravenous infusion of a parent’s Adipose-Derived Regenerative Cells (ADRCs), administered under the Federal Right to Try Act of 2017, improved developmental progress in four children with diverse diagnoses. [1] [2]
(Note: Quotes are summarized and lightly edited for clarity.)
Rasmussen’s Encephalitis
“Ann’s imagination has been back in full force. She would play, but it seems she’s more imaginative. Do you agree, Mom? “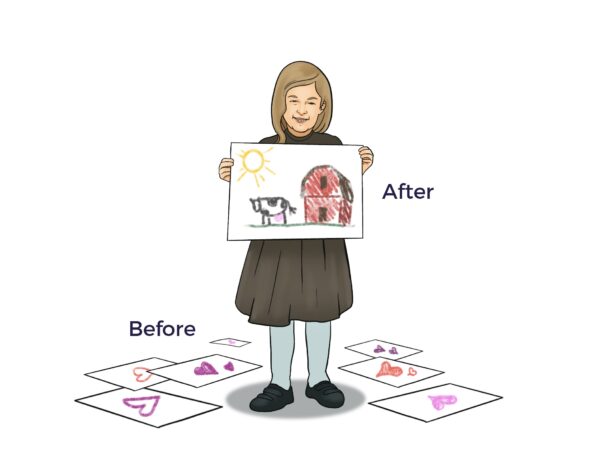
“Absolutely, the picture she made yesterday was really original…not the rote pictures of hearts she’s made the last months (love the love in the hearts, but it seemed she didn’t have access to the rest of her experience).”
Disorders of the Corpus Callosum
“Becky is walking all over the place. I let her decide where she is going unless we are running late, and then I hold her hand. She is doing so well.”
Rett Syndrome
“Sally has been very engaged. There are moments throughout the day when she is completely engaged, making eye contact and smiling, and she even finds things funny now.”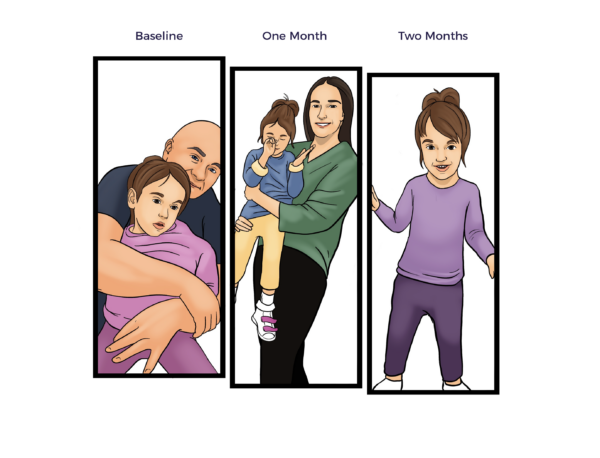
Idiopathic Autism
“Ashley is more complimentary and appreciative, overall, more alive!”
Common Symptoms – Different Diagnoses – A New Cell-Assisted Therapeutic Approach
“Autism, or autism spectrum disorder (ASD), refers to a broad range of conditions characterized by challenges with social skills, repetitive behaviors, speech and nonverbal communication,” according to Autism Speaks.
Adipose Tissue and the ADRCs residing within it contain a heretofore well-kept secret that prophesies a new cell therapy-based standard of care for ASD. More on that in a moment; first, let’s demystify developmental disabilities.
Contrary to conventional thought, a growing body of scientific evidence indicates that the Central Nervous System (CNS) alone does not cause ASD symptoms. Instead, researchers connect multiple body systems with developmental delays, seizures, and related health issues.
“Many behaviors and physiologic imbalances are common in secondary or known-cause autism and idiopathic or unknown-cause autism.” (Casanova et al. 2020)[3]
ASD stymies neurologists, pediatricians, and basic scientists for an apparent reason: It involves not just one spectrum but also sub-spectrums, sub-sub-spectrums, and so on.
For example, “Rett syndrome is caused by mutations on the X chromosome on a gene called MECP2. There are more than 900 different mutations found on the MECP2 gene, most found in eight different ‘hot spots…’, explains the International Rett Syndrome Foundation.
 Yet pharma and biotech focus on their unvaried one drug, one disease (or symptom) model. ASD’s diversity of causes and range of symptoms makes that approach unworkable. Additionally, the prescribed drugs often come with black box warnings, a multitude of potential side effects, and unpredictable interactions. [4] [5] [6]
Yet pharma and biotech focus on their unvaried one drug, one disease (or symptom) model. ASD’s diversity of causes and range of symptoms makes that approach unworkable. Additionally, the prescribed drugs often come with black box warnings, a multitude of potential side effects, and unpredictable interactions. [4] [5] [6]
Contrary to the drug development model, ADRCs are an “adipopharmacy.”
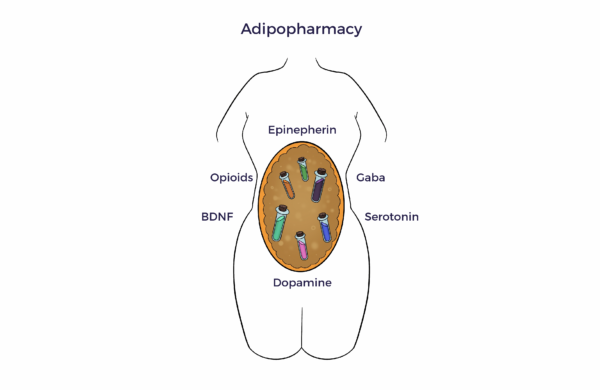
This mixed cell population uses natural intelligence (NI) to reverse the multiple dysregulations that cause the common ASD symptoms. From another angle, we have moved beyond the one-cell does-it-all paradigm. [7] [8] [9]
Notably, ADRCs “don’t care” about the cause, e.g., genetic, trauma, environmental, or brain malformation. Our subcutaneous fat doesn’t express these factors.
“ADRCs are smarter than we are; we just have to get out of the way and let them do their job,” said one prominent stem cell researcher.
HYPOTHESIS – LINKING AT WITH FUNCTIONAL IMPROVEMENT IN ASD
- A dissonance of physiologic dysregulations accounts for the common ASD symptoms and comorbidities. [10]
- Adipose tissue helps conduct our systemic symphony.
- ADRCs play the score by secreting hundreds of trophic (nutritional) factors (the secretome).
- The secretome restores multisystem harmony regardless of what instruments or section caused the body’s orchestra to play out of tune, e.g., a genetic mutation, environmental factors, congenital tissue malformations, or a combination thereof.
- Through neighboring cell-to-cell communication (the paracrine effect), ADRCs reharmonize the neurochemical imbalances that contribute to brain fog, lack of visual focus, anxiety, depression, seizures, and pain.[11] [12]
Just as music evokes an appropriate emotional response, a trend toward neurochemical balance and multisystem homeostasis, by definition, improves the quality of life for people with ASD-related functional deficits.
Ambrose Cell Therapy for ASD takes advantage of ADRCs’ lead in keeping subcutaneous adipose tissue healthy, even later in life. The fact that we can maintain or gain weight when we are older proves this point. This attribute is undeterred by chronic diseases, genetics, and environmental factors.[13] [14] [15]
ADIPOSE Tissue: The Body’s Pipe Organ—
 “In my eyes and ears, the organ is the King of Instruments.”
“In my eyes and ears, the organ is the King of Instruments.”
– Wolfgang Amadeus Mozart
A January 2024 PubMed search discovered over 145,000 papers on the science of adipose tissue. An understanding of the literature concludes that adipose tissue is the “pipe organ” of the body, including the brain.
Like the pipe organ imitates strings, horns, percussion, or wind instruments, adipose tissue (AT) functions as at least three bona fide organs.
- Endocrine—AT secretes hormones that control nutritional intake, metabolism, sexual function, fertility, immunity, vascular health, and more.
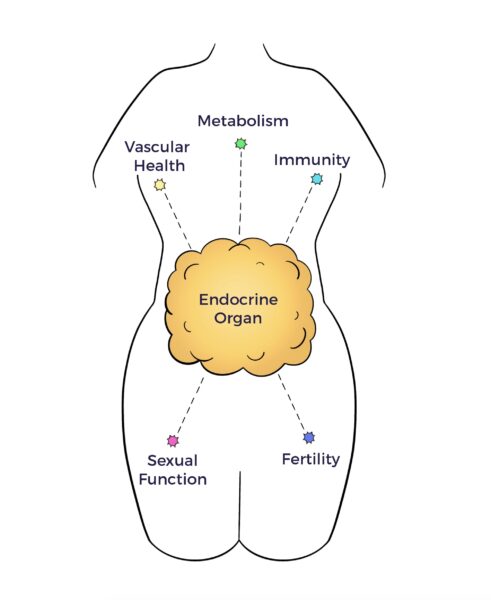
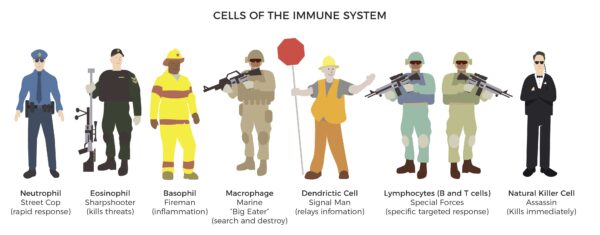
- Immune – AT contains all the immune cells of the body.
- Neurologic – AT releases neurochemicals to the brain and other organs. [16]
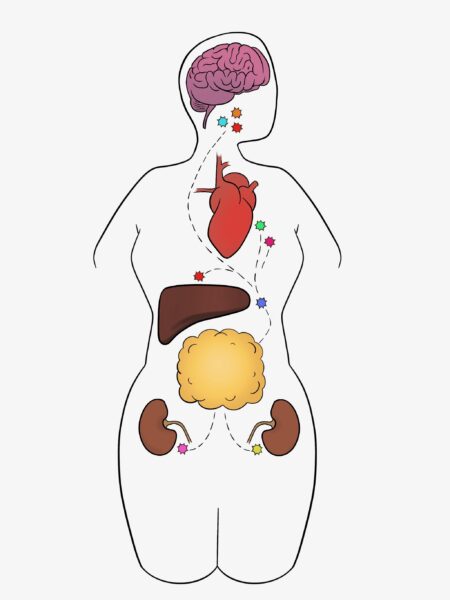
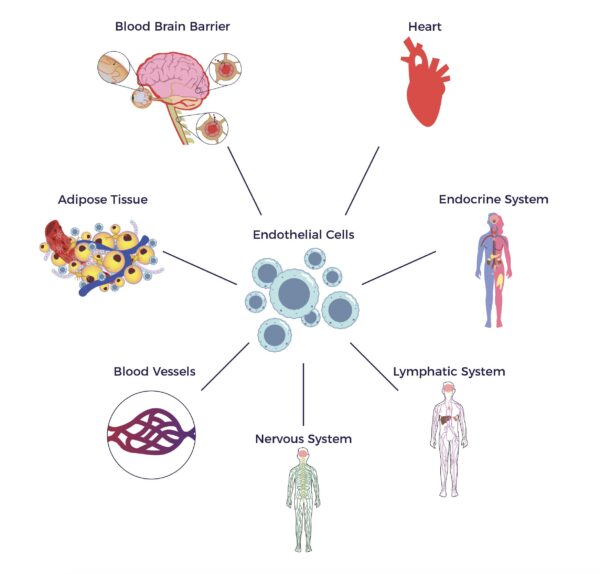
From another angle, AT contains endothelial cells and endothelial progenitor cells (EPCs), which stimulate blood cell formation and vascular and immune health, all factors that contribute to neurologic health. [17]
 In 2001, UCLA and University of Pittsburgh researchers unveiled a pool of multipotent cells in adipose tissue.[18] (Zuk et al. 2001) More than twenty years of research and over 115,000 publications followed their seminal paper.
In 2001, UCLA and University of Pittsburgh researchers unveiled a pool of multipotent cells in adipose tissue.[18] (Zuk et al. 2001) More than twenty years of research and over 115,000 publications followed their seminal paper.
Did the investigators imagine their discovery would lead to a brighter future for Ann, Ashley, Sally, Becky, and others with developmental disorders?
Uncommon Causes – A Common Connection
Peer-reviewed literature confirms an unexpected set of factors cause or contribute to ASD functional deficits, seizures, or inappropriate behaviors.
“The classical approach to autism spectrum disorders (ASD) is often limited to considering their neuro-functional aspects. However, recent scientific literature has shown that ASDs also affect many body systems and apparatuses such as the immune system, the sensory-motor system, and the gut-brain axis. The connective tissue, a common thread linking all these structures, may have a pathogenetic role in the multisystem involvement of ASD.” (Zoccante et al Feb. 2022) [19]
Connective tissue supports and protects our body’s systems and organs. However, connective tissue abnormalities lie at the heart of a wide range of diseases. In his paper, Dr. Zoccante coined the term “connectiviome” and included ASD in the connective tissue disorder spectrum.
The connectiviome theory begs the question: Can ADRCs that keep adipose connective tissue healthy be repurposed for treating ASD? But first, what other body systems connect to developmental delays?
THE AUTONOMIC NERVOUS SYSTEM AND THE CONNECTIVEIOME
“Autism spectrum disorder (ASD) is associated with atypical autonomic nervous system (ANS) function.” (Taylor et al. 2021) [20]
Your autonomic (self-governing) nervous system controls unconscious processes such as breathing, heart rate, blood pressure, muscle control, and bladder and bowel function.
The ANS consists of two nervous systems that supply or restrain nervous energy (innervate):
- The sympathetic nervous system (SNS) stimulates your fight-or-flight (stress) response.
- Through the vagus nerve, the parasympathetic nervous system (PNS) inhibits your SNS, allowing you to “rest and digest.”
In other words, the sympathetic and parasympathetic divisions work in concert to stimulate or inhibit various body processes. Their contrary but complementary functions help maintain whole-body balance or homeostasis, including in the gut.
Our SNS and PNS supply nerves to (innervate) connective tissue, including subcutaneous fat. Therefore, connective tissue health and disease are linked with ANS function or dysfunction.
Therein lies another notable connection between healthy fat and our ADRCs’ potential to retune the autonomic nervous system.
ADRCS RESTORE ANS BALANCE
Before cell therapy, Ashley and Ann displayed aggressive and repetitive behaviors, symptoms of sympathetic nervous system (SNS) hyper-arousal.
Following their intravenous delivery of ADRCs (ADRC-IV), their families shared their girl’s sustained behavioral improvements:
- “Ashley’s aggressive behavior is gone after her treatment,” says her mom.
- “Ann’s aunt noticed she wasn’t aggressive like she was before her cell therapy.”
- Ashley’s mom says she is joining her family and friends in conversations.
- Sally’s mom says she is “engaged.”
ADRCs and the Second Brain – The Gut Microbiome
Notably, the Vagus Nerve (VN), which extends from the base of the brain to the stomach, regulates the gut-brain axis. Thus, by reactivating the VN (restoring vagal tone), ADRCs also restore gut microbiome homeostasis. [21]
“Ann’s constipation is better,” reported her mother four months after ADRC therapy.
These dramatic behavior changes indicate that their girls’ parasympathetic nervous systems are more in tune with their environment. In addition, ADRCs restored Ann’s brain-gut-adipose-tissue communication pathways.[22]
Autonomic and Autoimmune Dysregulation
Many people believe inflammation is the root of all physical and emotional evils. However, there is more to it than that.
An out-of-control autonomic stress response raises cortisol and adrenaline levels, lighting the inflammation fire. Then, the immune system throws gas on the flames, resulting in autoimmune diseases. [23]
UC Davis Mind Institute studies find that ASD children have reduced immune system regulation, as well as shifts in their gut microbiome. A family history of autoimmune diseases adds fuel to the multisystem dysregulation blaze. [24] [25] [26]
In sum, the connectiviome, autonomic dysfunction, and autoimmune dysregulation disconnect the CNS and other systems of the developmentally delayed. [27] There is still more to the multisystem dysregulation cacophony.
ADSC’s immunomodulation mechanism plays a crucial role. They interact with immune cells, including T cells, B cells, macrophages, and dendritic cells, to modulate the immune response. [28]
Neurochemical Imbalances
The symphony’s conductor directs the musicians so that their performance enlivens our emotions. However, while only one conductor leads a symphony, a group of conductors called neurochemicals orchestrates our brains and peripheral and autonomic nervous systems.
Strikingly, over 40 neurochemicals guide our nervous systems. Then, our nerves direct our muscles and organs.
Research has identified altered neurochemical pathways involved in Rett Syndrome, epilepsy, brain injury, and other diagnoses along the continuum. [29]
“In this review, we aim to delineate the state-of-the-art main research findings about the neurochemical alterations in autism etiology…” (Marotta et al. 2020) [30]
ADIPOSE TISSUE – OUR THIRD BRAIN
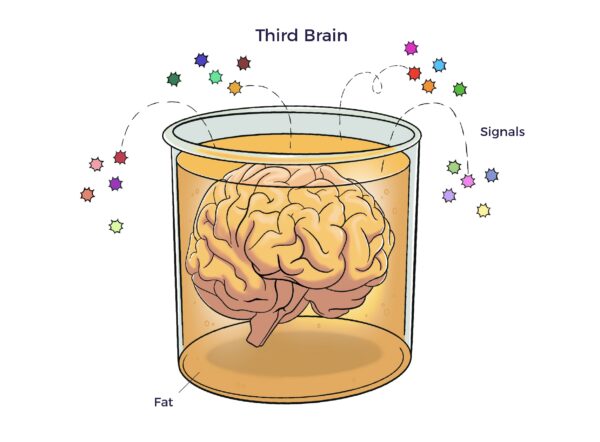 The adipose tissue as a third brain (Chaldakov et al. 2009) connects the adipose tissue secretome with neurochemical, ANS, and multisystem harmony.
The adipose tissue as a third brain (Chaldakov et al. 2009) connects the adipose tissue secretome with neurochemical, ANS, and multisystem harmony.
“Altogether, this may, like neuroendocrinology and neuroimmunology, open a novel field of research, neuroadipobiology. Its development may help humans stay lean, thoughtful, and noble.” (Emphasis added) [31]

The literature suggests that ADRCs harness neuroadipobiology and reverse “irreversible” neurochemistry.
Neurotrophic Factors (NF)
As a conductor directs the orchestra, NFs orchestrate our neurons’ growth, survival, and repair. They feed molecular nutrients to the brain, nervous, vascular, and immune systems. [32]
Examples:
- Brain-derived neurotrophic factor (BDNF) plays an essential role in neuroplasticity and neurodevelopment.[33]
- Insulin-like growth factor -1 (IGF-1) helps kids grow.
ADRCs increase NF levels
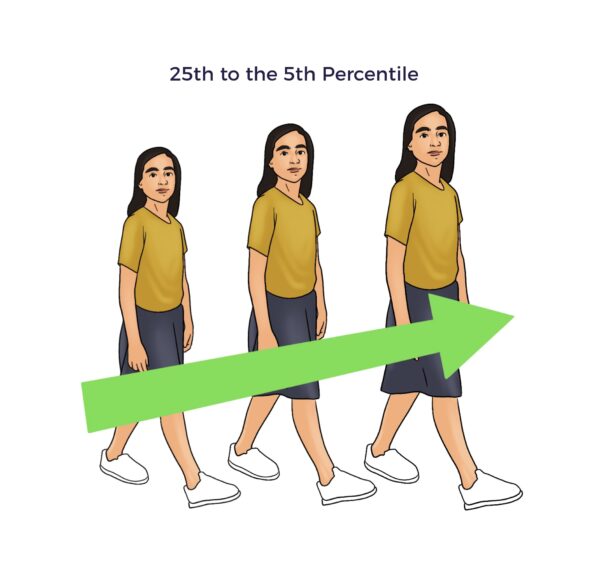
Ashley was at the 25th percentile of weight for her age, indicative of a lack of IGF-1 (insulin-like growth factor-1). She was also behind in school, suggesting reduced BDNF levels. [34] [35]
Within several months of her cell therapy, Ashley:
- Weighed in the 95th percentile, or average.[36]
- Graduated second grade with 100% final exam scores.
Ann’s reading level “improved by a whole grade” in the first five months of sixth grade,” her mom said.

Sally’s BMI (body mass index) was at the 15th percentile before her treatment. Four months after her treatment, she also reached the 95th percentile.

Ashley’s, Ann’s, and Sally’s restored development after extended delays suggests that their respective parents’ ADRCs increased IGF-1, BDNF, and other NFs.
Cell biology literature supports this theory.[37] [38] [39] [40] [41]
Neurotransmitters
Neurotransmitters carry messages from one nerve cell across to the next nerve, muscle, or gland cell, explains the Cleveland Clinic.
Dozens of neurotransmitters riff back and forth, depending on the tone of life’s music, like a jazz quartet in a jam session. Their proper balance creates a sense of well-being, energy, restfulness, excitement, relaxation, and so forth.
Example:
Gamma-aminobutyric acid (GABA) calms. Think of GABA as a mellow tune that helps you relax. On the opposite side of the coin, glutamate plays dance music. But if the music blares, GABA levels reduce, and glutamate increases.
- Reduced GABA influences visual perception, which could explain Ashley’s lazy eye and Becky’s lack of visual focus. [42] [43] [44]
After cell therapy, their moms shared:
Neuropeptide Y (NPY) helps harmonize the central and peripheral nervous systems. It plays alongside GABA and glutamate. NPY’s functions include controlling epileptic seizures. Research has shown a significant reduction in NPY levels during the delayed post-seizure recovery (postictal period). [47]
- Sally had a history of fever-induced seizures (febrile seizures). A month after her ADRC infusion, she caught a high fever and a UTI. This time, “No seizures,” reported her mom.
- Ten months after Ann received ADRCs, her mother and grandmother noted a significant reduction in seizures, and that when “Ann does have a glitch, she recovers right away. In fact, she talked right through one,” noted her mother.
Lest we undervalue Ann’s dramatic improvement, even if imperfect, several seizures per day or “seizure clusters” increase the risk of sudden unexplained death (SUND) by 2.5 times.[48]
Neuroendocrine hormones
“The central neuroendocrine systems are responsible for the control of homeostatic processes in the body, including reproduction, growth, metabolism and energy balance, and stress responsiveness.” (Gore 2010) [49]
Example:
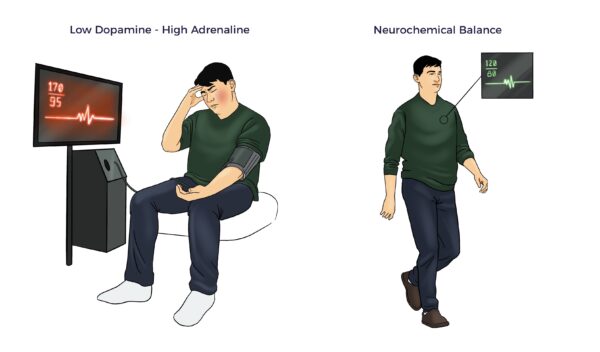
Dopamine, norepinephrine, and epinephrine maintain homeostasis through the autonomic nervous system.
- Epinephrine (adrenaline), an adrenal hormone, takes over in acute stress. It increases inflammation, raises your heart rate and blood pressure, etc.
- When the stress subsides, dopamine brings a sense of pleasure and reward.
This continuous balancing act between dopamine and adrenal hormones also plays a role in your fight-or-flight response.
After ADRC therapy:
- Ann had a setback with her annual flu shot. It took about a week, but her mom messaged, “Ann is back to her bubbly self.”
- “Ashley is joining conversations. She never did that before,” said her mom.
- “Sally’s dad dropped something on the floor. She picked it up and handed it back to her dad!”. Her mom said this was the first time Sally had demonstrated initiative with perfect motor control. The family started singing “stem cells, stem cells, stem cells” in recognition of Sally’s new ability.
Neurochemical Summary
ADRCs’ treasure trove of signaling molecules, hormones, growth factors, and neurotrophic factors helps:
- Rebalance the brain’s neurochemicals.
- Reduce neuroinflammation and immune dysregulation.
- Rehabilitate neuroplasticity.
- Improve ANS function and vagal tone.
- Improve blood flow and glucose metabolism in the brain. [50]
- Restore multisystem homeostasis.[51] [52] [53] [54] [55]
Conclusion
Ambrose Cell Therapy has demonstrated with four ASD patients with different diagnoses that a single infusion of a parent’s ADRCs made a profound difference in their developmental progress and quality of life. These strong signals of safety and effectiveness call for continued investigation and therapy under the Federal Right to Try Act of 2017.
[2] Autologous Donors of Blood and Blood Components Intended Solely for Autologous Use – Compliance Policy
[3] Casanova MF et al. Editorial: Secondary vs. Idiopathic Autism. Front Psychiatry. 2020 Apr 14;11:297.
[4] Clarke C, Evans J, Brogan K. Treatment Emergent Violence To Self And Others; A Literature Review of Neuropsychiatric Adverse Reactions For Antidepressant And Neuroleptic Psychiatric Drugs And General Medications. Adv Mind Body Med. 2019 Winter;33(1):4-21.
[5] Moore TJ et al. (2010) Prescription Drugs Associated with Reports of Violence Towards Others. PLoS ONE 5(12): e15337.
[6] Spencer D et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013 Nov;132(5):833-40. doi: 10.1542/peds.2012-3774. Epub 2013 Oct 21. PMID: 24144704; PMCID: PMC3813388.
[7] Alshoubaki YK et al. Modulation of the Activity of Stem and Progenitor Cells by Immune Cells. Stem Cells Transl Med. 2022 Mar 31;11(3):248-258
[8] Caplan AI. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med. 2017 Jun;6(6):1445-1451.
[9] Zenić L et al. Medicinal signaling cells niche in stromal vascular fraction from lipoaspirate and microfragmented counterpart. Croat Med J. 2022 Jun 22;63(3):265-272.
[10] Chugani, Diane C et al. Autism Spectrum Disorders (New York, 2011; online edn, Oxford Academic, 1 Sept. 2012),
[11] Zoccante L et al. The “Connectivome Theory”: A New Model to Understand Autism Spectrum Disorders. Front Psychiatry. 2022 Feb 7;12:794516.
[12] Booth A et al. Adipose tissue: an endocrine organ playing a role in metabolic regulation. Horm Mol Biol Clin Investig. 2016 Apr 1;26(1):25-42.
[13] Perin EC, Willerson JT. Buying new soul. J Am Coll Cardiol. 2012 Nov 20;60(21):2250-1.
[14] Schmitz C et al. The Composition of Adipose-Derived Regenerative Cells Isolated from Lipoaspirate Using a Point of Care System Does Not Depend on the Subject’s Individual Age, Sex, Body Mass Index and Ethnicity. Cells. 2022 Dec 21;12(1):30.
[15] Trevor, L.V.; Riches-Suman, K.; Mahajan, A.L.; Thornton, M.J. Stromal Vascular Fraction Cells from Individuals Who Have Previously Undergone Radiotherapy Retain Their Pro-Wound Healing Properties. J. Clin. Med. 2023, 12, 2052.
[16] Parimisetty A et al. . Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J Neuroinflammation. 2016 Mar 24;13(1):67.
[17] Han J et al. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010 Feb 4;115(5):957-64.
[18]Zuk PA et al. Multilineage cells from human adipose tissue: implications for cell based therapies.Tissue Eng. 2001;7:211–27.
[19] Zoccante L et al. The “Connectivome Theory”: A New Model to Understand Autism Spectrum Disorders. Front Psychiatry. 2022 Feb 7;12:794516.
[20] Taylor, E. C., Livingston, L. A., Callan, M. J., Ashwin, C., & Shah, P. (2021). Autonomic dysfunction in autism: The roles of anxiety, depression, and stress. Autism, 25(3), 744-752
[21] Breit S et al. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front Psychiatry. 2018 Mar 13;9:44.
[22] Yi CX, Tschöp MH. Brain-gut-adipose-tissue communication pathways at a glance. Dis Model Mech. 2012 Sep;5(5):583-7.
[23] Bellocchi C et al. The Interplay between Autonomic Nervous System and Inflammation across Systemic Autoimmune Diseases. Int J Mol Sci. 2022 Feb 23;23(5):2449.
[24] Wu S et al. Family history of autoimmune diseases is associated with an increased risk of autism in children: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2015 Aug;55:322-32.
[25] Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012 Mar;26(3):383-92.
[26] Hughes HK et al. Immune Dysfunction and Autoimmunity as Pathological Mechanisms in Autism Spectrum Disorders. Front Cell Neurosci. 2018 Nov 13;12:405.
[27] Salari V et al. The Anti-Inflammatory Properties of Mesenchymal Stem Cells in Epilepsy: Possible Treatments and Future Perspectives. Int J Mol Sci. 2020 Dec 18;21(24):9683.
[28] Ceccarelli S et al. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front Cell Dev Biol. 2020 Apr 17;8:236.
[29] Cetin F H et al. ‘Neurotransmitter Systems in Autism Spectrum Disorder’, Autism Spectrum Disorder – Recent Advances. InTech, Apr. 02, 2015.
[30] Marotta R et al. The Neurochemistry of Autism. Brain Sci. 2020 Mar 13;10(3):163.
[31] Chaldakov G et al. (2009). The adipose tissue as a third brain. Obesity and Metabolism. 5. 94-96.
[32] Kermani P, Hempstead B. BDNF Actions in the Cardiovascular System: Roles in Development, Adulthood and Response to Injury. Front Physiol. 2019 Apr 26;10:455.
[33] Erdoğan, M. & Erbas, Oytun. (2023). The Role of Brain-Derived Neurotrophic Factor in Autism Spectrum Disorder: Current Findings and Future Directions. 10.5772/intechopen.112471.
[34] Kahathuduwa, Chanaka N., et al. “Autism spectrum disorder is associated with an increased risk of development of underweight in children and adolescents: A systematic review and meta-analysis.” Research in Autism Spectrum Disorders94 (2022): 101969.
[35] Erdoğan, M. & Erbas, Oytun. (2023). The Role of Brain-Derived Neurotrophic Factor in Autism Spectrum Disorder: Current Findings and Future Directions. 10.5772/intechopen.112471.
[36] Wrigley S, Arafa D and Tropea D (2017) Insulin-Like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front. Cell. Neurosci. 11:14.
[37] Kerschensteiner M et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999 Mar 1;189(5):865-70.
[38] Clauser et al. Adipose-derived stem cells secrete neurotrophic factors. Annals of Oral & Maxillofacial Surgery 2013 Mar 01;1(2):12
[39] Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther. 2016 Sep 9;7(1):131. doi: 10.1186/s13287-016-0394-0.
[40] Bagno LL et al. Sustained IGF-1 Secretion by Adipose-Derived Stem Cells Improves Infarcted Heart Function. Cell Transplant. 2016;25(9):1609-1622.
[41] Pak J et al. (2020) Potential Benefits of Allogeneic Haploidentical Adipose Tissue-Derived Stromal Vascular Fraction in a Hutchinson–Gilford Progeria Syndrome Patient. Front. Bioeng. Biotechnol. 8:574010.
[42] Blue ME, Naidu S, Johnston MV. Altered development of glutamate and GABA receptors in the basal ganglia of girls with Rett syndrome. Exp Neurol. 1999 Apr;156(2):345-52.
[43] Song C et al. Human Occipital and Parietal GABA Selectively Influence Visual Perception of Orientation and Size. J Neurosci. 2017 Sep 13;37(37):8929-8937.
[44] Braat S, Kooy RF. The GABAA Receptor as a Therapeutic Target for Neurodevelopmental Disorders. Neuron. 2015 Jun 3;86(5):1119-30.
[45] Zhu F et al. (2019). The GABA receptor GABRR1 is expressed on and functional in hematopoietic stem cells and megakaryocyte progenitors. Proceedings of the National Academy of Sciences. 116. 201906251. 10.1073/pnas.1906251116.
[46] Bhandage AK, Barragan A. GABAergic signaling by cells of the immune system: more the rule than the exception. Cell Mol Life Sci. 2021 Aug;78(15):5667-5679.
[47] McGuire JL et al. Differential Regulation of Neuropeptide Y in the Amygdala and Prefrontal Cortex during Recovery from Chronic Variable Stress. Front Behav Neurosci. 2011 Sep 15;5:54.
[48] Bauman K, Devinsky O. Seizure Clusters: Morbidity and Mortality. Front Neurol. 2021 Feb 16;12:636045.
[49] Gore AC. Neuroendocrine targets of endocrine disruptors. Hormones (Athens). 2010 Jan-Mar;9(1):16-27.
[50] Wang Y, Yu S, Li M. Neurovascular crosstalk and cerebrovascular alterations: an underestimated therapeutic target in autism spectrum disorders. Front Cell Neurosci. 2023 Aug 24;17:1226580.
[51] Lina Badimon, Judit Cubedo, Adipose tissue depots and inflammation: effects on plasticity and resident mesenchymal stem cell function, Cardiovascular Research, Volume 113, Issue 9, July 2017, Pages 1064–1073
[52] Naik S, Larsen SB, Cowley CJ, Fuchs E. Two to Tango: Dialog between Immunity and Stem Cells in Health and Disease. Cell. 2018 Nov 1;175(4):908-920.
[53] Alshoubaki YK et al. Modulation of the Activity of Stem and Progenitor Cells by Immune Cells. Stem Cells Transl Med. 2022 Mar 31;11(3):248-258
[54] Sarlo GL, Holton KF. Brain concentrations of glutamate and GABA in human epilepsy: A review. Seizure. 2021 Oct;91:213-227.
[55] Marotta R et al. The Neurochemistry of Autism. Brain Sci. 2020 Mar 13;10(3):163.
