Rationale for Spinal Cord Injury Cell Therapy
In 2006, a group of researchers from Korea and Canada wrote a review paper covering a number of stem cell studies in small and large animals for spinal cord injury (SCI). They noted the potential for adult stem cell therapy to improve function in SCI patients. As the research has progressed, human trials using adult stem cells have added support to that thesis. [1] [2] [3] [4]
Fast forward to late 2019, Mayo Clinic published a case report, in which stem cells from a patient’s fat (adipose-stem cells or ASCs) were delivered via the spinal canal (intrathecally) to an SCI patient. The one-time treatment resulted in a remarkable improvement in function and symptoms. Notwithstanding the fact that the report was for a single patient (selected from a larger trial), the author referred to the promise this “super-responder” represents for those suffering similarly. [5]
From another angle, positive cell therapy studies or case reports have also been published that relate to some of the follow-on effects that can result from the injury to the spinal cord or subsequent surgeries. The reported benefits include, but are not limited to, reduction of scar and neuropathic pain as well as improvement in bowel, bladder and sexual functions. [6] [7]
ADRCs
AMBROSE accesses the mixed population of stem and regenerative cells in a patient’s fat (adipose tissue). This cell preparation, when clinical grade, is called adipose-derived stem and regenerative cells (ADRCs). [8]
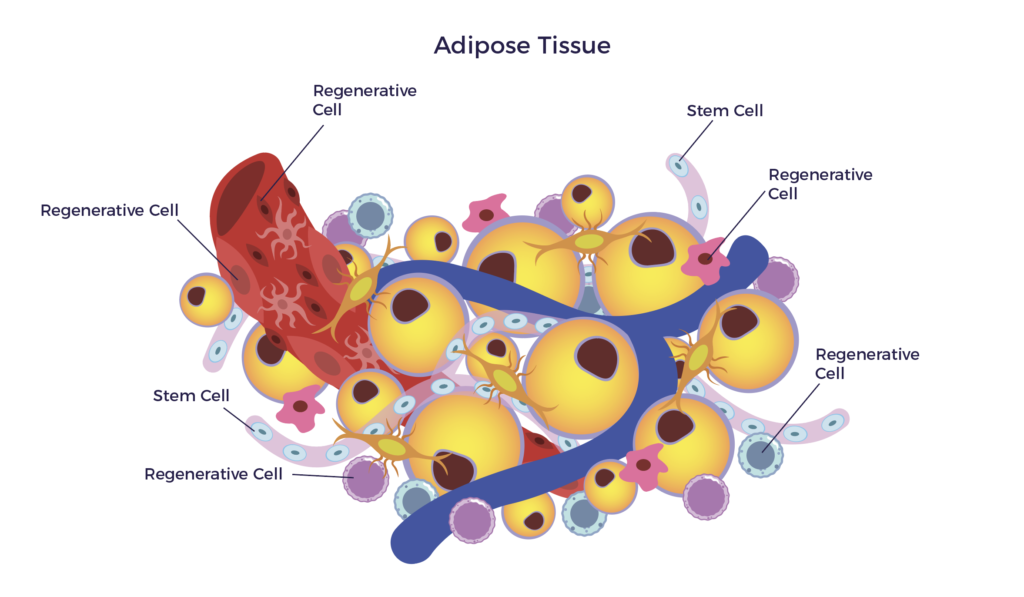
ADRCs are akin to having your own, personalized fire department. They wait quietly for a sign of trouble – inflammation – and then home to that site to do their jobs. When they arrive on the scene, they blanket a diseased tissue or organ with hundreds of biologically active molecules that promote cellular, nerve and tissue repair. [9] More on the process of repair in a moment; first some basics:
Spinal Cord Injury
The brain and spinal cord comprise the central nervous system. Sensory and motor nerve impulses travel to and from the brain along the nerve fibers that make up the spinal cord. This long tube-like structure runs from the base of the brain to the lower back (lumbar) region. These bundled nerve fibers are protectively surrounded by the bones of the vertebra. Injury to a vertebral bone can tear or compress the spinal cord, which can impact sensation and function.
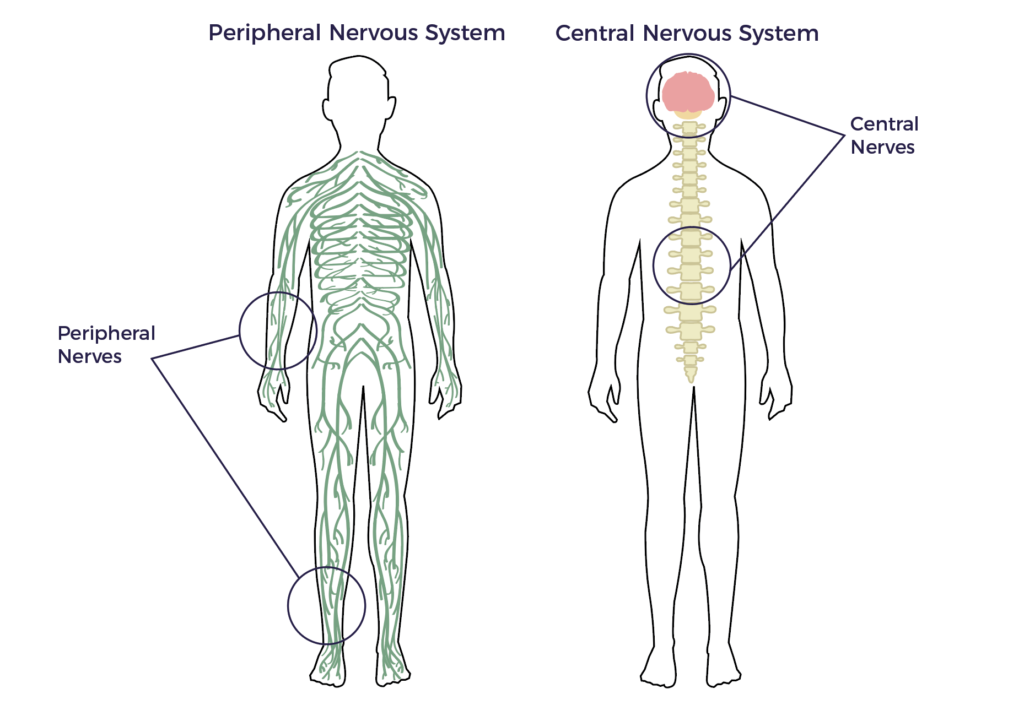
Different degrees of paralysis depend on the location and severity of the damage. Loss of nerve cells (neurons) may also affect talking, breathing and memory in various ways and at different time points. This all depends on the affected areas of the CNS, the degree of neuroinflammation and rate of degeneration that follows.
SCI After-Effects
The after-effects of traumatic spinal cord injury (SCI) are clearly significant and often catastrophic. While what the observer sees is the paralysis for those most affected, it can also mean pain as well as a host of other less obvious disabilities. In other words, the consequences of SCI are not confined to the spinal cord; other parts and systems of the body are typically involved.
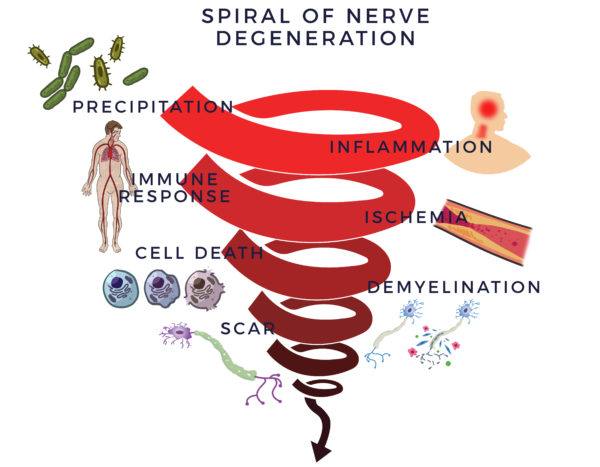
Spiral of Degeneration
The trauma from the impact on the spinal cord leads to neuroinflammation, defined as chronic inflammation in the central nervous system (CNS). This causes the immune system to overreact (inflammatory-immune response) to protect the nerves. Unfortunately, this is like a backseat driver thinking they are helping you but instead they cause you to get in a car wreck. This response leads to poor blood flow (ischemia) in the spinal cord. Our CNS needs blood flow to bring oxygen and nutrients to it to keep it healthy. Without it, the cells of the myelin sheathing, which insulate the nerves, die off, scar forms and the CNS stops functioning normally. We call this the Spiral of Degeneration.
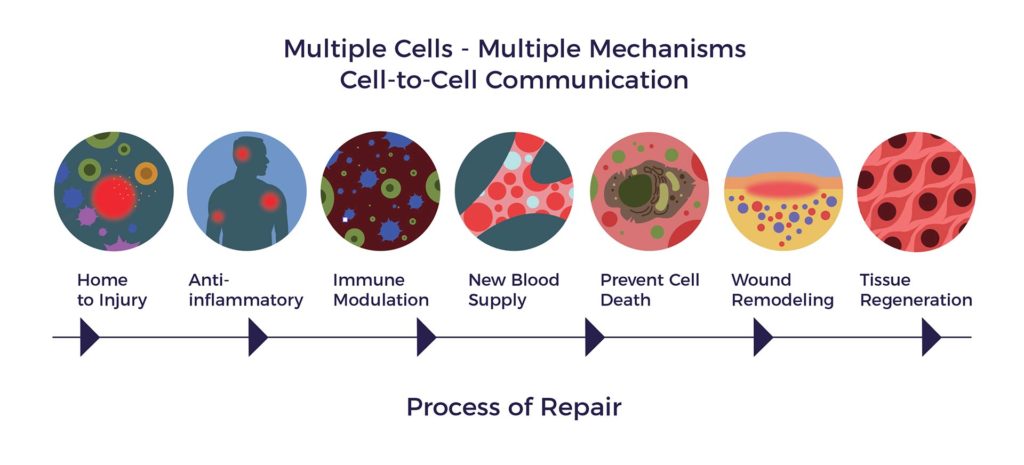
Process of Repair
On the other side of the coin, ADRCs, through cell-to-cell communication, mobilize nearby cells to go back to work and do their jobs of repair. This is called the “paracrine effect”.
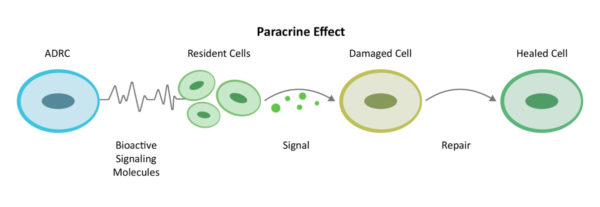
ADRCs first decrease inflammation and the overactive immune response. Once the backseat driving has diminished, they increase circulation with new blood vessel growth, prevent further programmed cell death, decrease scar size – or in the case of SCI remyelinate the nerves – and regenerate. We call this the Process of Repair.
Neurotrophic Factors
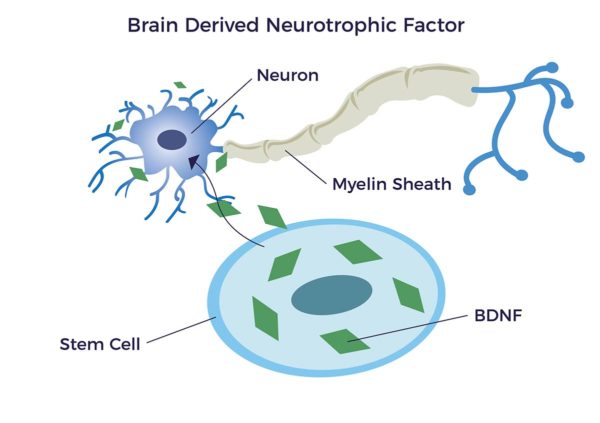
Very relevant and important to SCI repair are a family of biomolecules that could be thought of as an organic fertilizer for our nerves. These are called “Neurotrophic factors (NTFs)”. Neuro relating to nerve and trophic is from Ancient Greek τροφικός (trophikós) meaning “pertaining to food or nourishment”. NTFs support the growth, survival, and differentiation of both developing and mature nerve cells (neurons), including new myelin sheathing (remyelination).
It has been documented that adipose-derived stem cells (ADSCs) specifically release brain-derived neurotrophic growth factor (BDNF). BDNF promotes nerve healing, remyelination and axon growth. [10] [11]
To summarize, ADRCs have a range of potential activities including but not limited to:
• Homing toward scarred (lesioned) areas
• Decreasing spinal cord inflammation
• Differentiating into neurons (neurogenesis)
• Promoting nervous tissue maintenance and repair
• Reducing disease severity
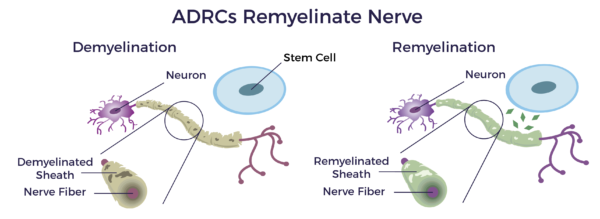
• Decreasing demyelination
• Remyelination [12] [13] [14]
Patient Expectations
The ASIA Impairment Scale (AIS) ranges from A to E, where A is a complete SCI and E denotes normal sensory and motor function. A patient’s AISA score is a monitoring factor in the expectations one should have for improvement. Said another way, the goal of AMBROSE Cell Therapy is improvement in symptoms, function and quality of life over the baseline AIS score.
[1] B.G. Kim et al Stem Cell-Based Cell Therapy for Spinal Cord Injury Cell Transplantation, 16(4), 355–364 2007
[2] Mendonça et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury Stem Cell Research & Therapy 2014, 5:126
[3] JW Hur et al. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J Spinal Cord Med. 2016;39(6):655–664.
[4] Y Kim et al. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury Stem Cell Research & Therapy (2015) 6:229
[6] M Bydon et al. CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury Mayo Clinic Proceedings, Volume 0, Issue 0
[6] GL Nanninga et al. Lipofilling may induce nerve regeneration after previous traumatic injury: a clinical case with remarkable outcome Eur J Plast Surg (2016) 39:383–386
[7] Fat Grafting in Burn Scar Alleviates Neuropathic Pain via Anti-Inflammation Effect in Scar and Spinal Cord PLOS ONE September 14, 2015
[8] JK Fraser PhD and S. Kesten MD Autologous Adipose Derived Regenerative Cells: A platform for therapeutic applications Advanced Wound Healing Surgical Technology International XXIX
[9] A Caplan PhD MSCs: The Sentinel and Safe-Guards of Injury J. Cell. Physiol. 231: 1413–1416, 2016.
[10] Razavi, Shahnaz et al. “Neurotrophic Factors and Their Effects in the Treatment of Multiple Sclerosis.” Advanced Biomedical Research 4 (2015): 53. PMC. Web. 28 Sept. 2018.
[11] T Lopatina et al. (2011) Adipose-Derived Stem Cells Stimulate Regeneration of Peripheral Nerves: BDNF Secreted by These Cells Promotes Nerve Healing and Axon Growth De Novo. PLoS ONE 6(3): e178991
[12]AJ Mothe and CH Tator Advances in stem cell therapy for spinal cord injury J Clin Invest. 2012;122(11) :3824-3834
[13] Azim Hedayatpour, Ph.D. et al Promotion of remyelination by adipose mesenchymal stem cell transplantation in a cuprizone model of multiple sclerosis. Cell J. 2013; 15(2): 142-151.
[14] S. Seigo et al, Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration, Journal of Orthopaedic Science, Volume 18, Issue 1,2013, Pages 145-151
