Retinitis Pigmentosa Hypothesis
Summary
Here, we hypothesize that autologous Adipose-Derived Regenerative Cells (ADRCs) is a new option for patients living with retinitis pigmentosa. In contrast to a drug with a single mechanism of action, ADRCs regulate the multiple factors contributing to a patient’s loss of eyesight.
Background16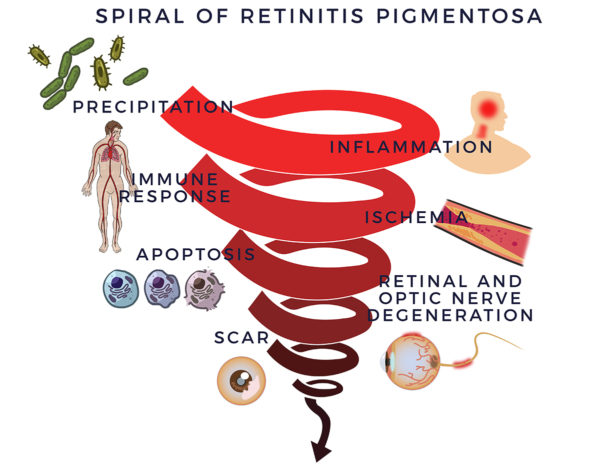 In 1857, Dr. Donders identified a group of incurable eye disorders he named retinitis pigmentosa (RP). But it was not as simple as that: Subsequently, researchers discovered over 100 genes that can contain mutations leading to retinitis pigmentosa. But genetic abnormalities do not clarify everything; half of RP cases lack previous family history and explanation.
In 1857, Dr. Donders identified a group of incurable eye disorders he named retinitis pigmentosa (RP). But it was not as simple as that: Subsequently, researchers discovered over 100 genes that can contain mutations leading to retinitis pigmentosa. But genetic abnormalities do not clarify everything; half of RP cases lack previous family history and explanation.
Yet, despite the disease’s complexity, able investigators have mapped out RP’s degenerative process. Retinitis means inflammation of the retina. That inflammation leads to a spiral of degeneration of the retina and optic nerve.
ADRC Repair Process
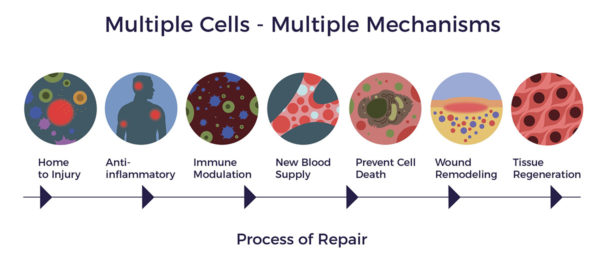
In the presence of infection, injury, or disease, ADRCs home to sites of inflammation and initiate a repair process through multiple mechanisms of action.
Time For a New Option Drug and gene therapy developers continue to pursue the failed “one molecule or gene for one disease model” for RP. This minimalist approach has not produced a drug or gene therapy that changes disease progression or improves patients’ best-corrected visual acuity (BCVA).
Drug and gene therapy developers continue to pursue the failed “one molecule or gene for one disease model” for RP. This minimalist approach has not produced a drug or gene therapy that changes disease progression or improves patients’ best-corrected visual acuity (BCVA).
In the real world, the 100,000 people in the US diagnosed with RP lack an option that slows, stabilizes, or reverses the disease’s progression. Instead, most are legally blind by age 40. Therefore, patients need a new standard of care regardless of the gene mutation or other culprit causing RP.
Hypothesis
Here, we hypothesize that autologous Adipose-Derived Regenerative Cells (ADRCs) is a new option for patients living with retinitis pigmentosa. In contrast to a drug with a single mechanism of action, ADRCs regulate the multiple factors contributing to a patient’s loss of eyesight.
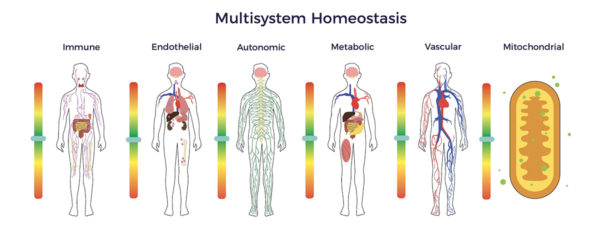
The cornerstone of our hypothesis is that all the body’s systems are interrelated and dependent. As such, multisystem dysfunction contributes to degenerative diseases, including RP [1] [2] [3] [4] [5]
Specifically, RP-related multisystem dysregulation results in:
- Oxidative stress,
- Reduced nitric oxide levels,
- Elevated systemic inflammation,
- Abnormal immune response,
- Metabolic dysregulation,
- Endothelial dysfunction and
- Mitochondrial impairment, and
- Autonomic dysfunction.[6] [7] [8] [9]
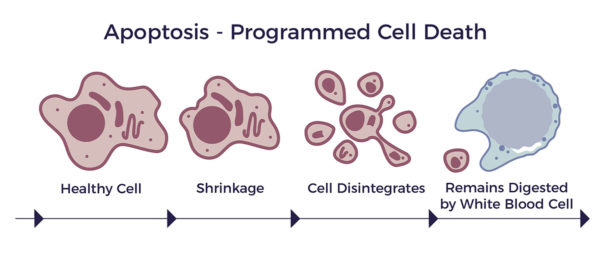
These abnormalities lead to apoptosis of the retinal photoreceptors and blindness. [10]
IV Protocol Rationale
We propose IV delivery of ADRCs based on:
- A rat study mimicking human RP demonstrated that IV infusion is superior to subretinal delivery. “It would appear that stem cells exert their effect over the whole retina when administered systemically. In comparison, subretinal delivery of cells including bone marrow-derived cells usually results in rod and cone rescue” (S. Wang 2010) [11]
- A human study of Umbilical Cord MSCs (UCMSCs) confirmed systemically-delivered stem cells’ feasibility, safety, and benefit. “Most patients improved their best-corrected visual acuity (BCVA) in the first three months. The proportions of patients with improved or maintained BCVA were 96.9%, 95.3%, 93.8%, 95.4%, 90.6%, and 90.6% at the 1st, 2nd, 3rd, 6th, 9th, and 12th-month follow-up, respectively. Most of the patients (81.3%) maintained or improved their visual acuities for 12 months.” (T. Zhao 2020). The researchers also proposed that the breakdown of the blood-retinal-barrier (BRB) in the progression of RP makes it possible that infused cells can reach the impaired retinal tissue without the need for injections directly in the eye. [12] [13] [14]
- A study of IV injection of USMSCs vs. direct injection of a steroid showed, “UCMSC intravenous infusion shows slow but persistent action in alleviating ME (macular edema) and can improve the visual function for a longer time.”.[15]
- A Japanese group compared the trophic factors secreted from fresh ADRCs vs. cultured ASCs (adipose-derived MSCs) from the same person. The study indicates that ADRCs are more multifunctional and potent than cultured ASCs cells. ADRCs released a greater variety of cytokines or soluble proteins at significantly higher amounts than ASCs. [16]
The favorable comparison noted above extends to MSCs derived from other sources such as bone marrow, umbilical cord, and placenta. - Conversely, several direct injection studies reported adverse events such as retinal tears and fibrosis. Therefore, both the safety profile and IV infusion pathways show advantages.
AMBROSE Protocol for Retinal Diseases:
- A board-certified plastic surgeon, using water-assisted liposuction (WAL), harvests 400 ccs of lipoaspirate. WAL is minimally traumatic on the patient and tissue, resulting in higher ADRC yields and viability.
- The Celution system liberates the ADRCs from the lipoaspirate.
- A nurse inserts an IV and delivers mannitol. Mannitol is a sugar alcohol that temporarily disrupts the BRB. It is a standard of care for delivering drugs into the back of the eye.
- The ADRCs are delivered intravenously over a 20-minute infusion.
The outpatient procedure takes about five hours. Of that time, cell preparation takes 2.5 hours, during which the patient rests comfortably.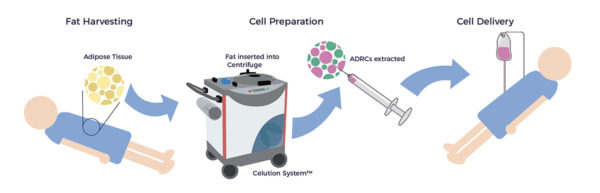
ADRCs
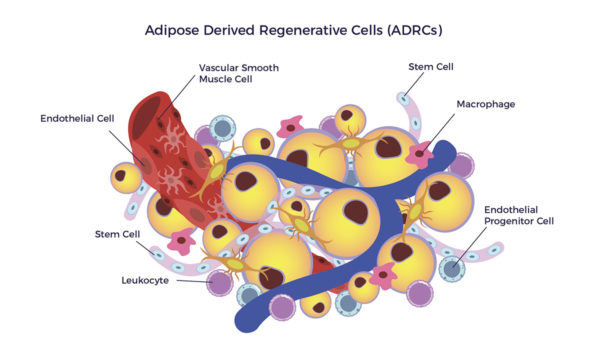
Adipose-Derived Regenerative Cells (ADRCs) is the designation for a clinical-grade preparation of stromal vascular fraction (SVF). ADRCs’ inherent role is maintaining cellular, tissue, and systemic homeostasis.[17] [18] [19]
 ADRCs are a heterogeneous population of cells, including mesenchymal stem cells, other progenitor cells, fibroblasts, T-regulatory cells, and macrophages. The mix includes a high percentage of endothelial cells, endothelial progenitor cells, macrophages, and leukocytes.
ADRCs are a heterogeneous population of cells, including mesenchymal stem cells, other progenitor cells, fibroblasts, T-regulatory cells, and macrophages. The mix includes a high percentage of endothelial cells, endothelial progenitor cells, macrophages, and leukocytes.
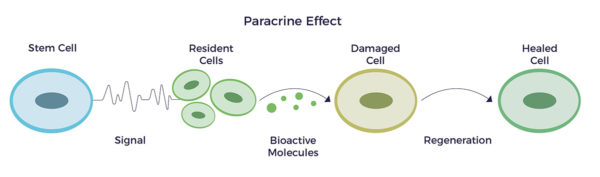
 After homing to an inflammatory signal, the ADRC-secretome releases hundreds of cytokines and growth factors to the diseased microenvironment. The endogenous cells send signals back to the ADRCs.
After homing to an inflammatory signal, the ADRC-secretome releases hundreds of cytokines and growth factors to the diseased microenvironment. The endogenous cells send signals back to the ADRCs.
This crosstalk instructs the individual cell types necessary for repair to activate – and those not needed (or harmful) to stand down. Put differently, the plethora of biological agents in the secretome restores cellular stability and homeostasis.
ADRCs – Miracle-Gro for Nerve Repair
Miracle-Gro feeds your garden’s soil with the nutrients it needs to grow healthy roots, stems, petals, and leaves. Just as there are situations in which we fertilize a plant lacking vital nutrients, ADRCs secrete growth factors essential to the health of our aging brains, hearts, muscles, nerves, and so on. [20]
One such growth factor group is “Neurotrophic factors (NTFs).” Neuro relating to nerve and trophic, from Ancient Greek trophikós, meaning “of food or nourishment.” In other words, NTFs feed our neurons and nerves with nutrients.
Notably, Brain-Derived Neurotrophic Growth Factor (BDNF) stimulates new neurons, nerve cell connections, and nerves. It also repairs the myelin sheathing surrounding the nerves. Further, this remarkable molecule is anti-inflammatory and anti-apoptotic. Diminished BDNF levels correlate with the progression of RP. [21] [22] [23] [24] [25]
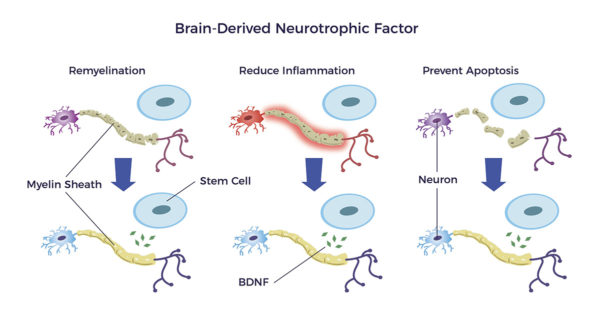
A recent discovery of neuroimmune cells in ADRCs is notable. Neuroimmune cells innervate tissues and release BDNF.[26] Additionally, other cytokines in adipose tissue secrete an abundance of neurogenerative factors.
Human studies show ADRCs release factors that:
- Down-regulate inflammatory-autoimmune markers, including but not limited to TNF-A and TH17,
- Reduce the production of Endothelin-1, a known constrictor of blood vessels and a culprit in subsets of RP patients.
- Include Placental Growth Factor (PGF), Stromal-Derived Factor-1 (SDF-1), and Vascular Endothelial Growth Factor (VEGF), all of which assist in the growth and stabilization of new blood vessels. These GFs are also anti-inflammatory and anti-apoptotic.
- Promote the switch from inflammatory macrophages (M1s) to anti-inflammatory macrophages (M2s) such as prostaglandin E2 (PGE2).
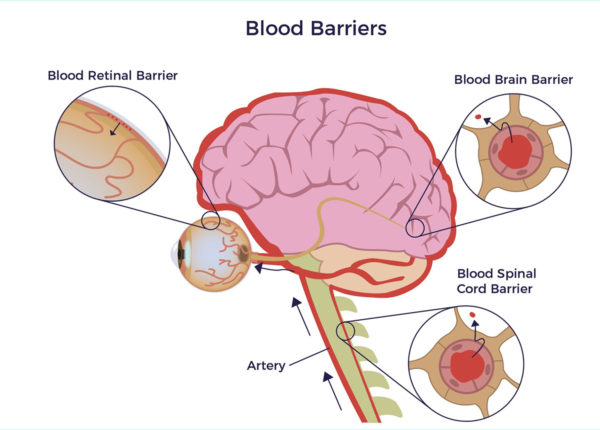 Permeating the Blood-Retinal-Barrier
Permeating the Blood-Retinal-Barrier
A critical concern of physicians and patients regarding ocular diseases is whether adult stem cells can be delivered non-invasively and safely permeate the blood-retinal-barrier (B-R-B), an extension of the blood-brain-barrier. The B-B-B protects our brains, eyes, and the spinal canal from microbial invaders. It is our central nervous system’s version of Fort Knox. Still, instead of a granite-lined concrete structure, epithelial and endothelial cells line the outer and inner blood-retinal-barrier, respectively. [27]
Three mechanisms allow the migration of MSCs and the ADRC secretome into the back of the eye.
- Mannitol, a safe sugar alcohol, temporarily opens the B-B-B.
- ADRCs release cytokines that permeate the B-B-B,
- MSCs possess the ability to cross the B-B-B. [28] [29]
As the lymphatic vessels parallel the vascular system, they may be a route that stem cells migrate from the spleen to bypass the blood-brain-barrier too. [30]
Age and ADRCs
Though aging is a failure of stem cells, ADRCs in the subcutaneous fat remain accessible, abundant, and potent throughout one’s life. (J. Willerson et al. Buying New Soul 2013) [31] Thus, autologous ADRCs are effective in elderly patients.
Safety
The Celution System® is a closed, sterile lab-in-a-box. Celution liberates autologous clinical-grade ADRCs from lipoaspirate at the point of care.
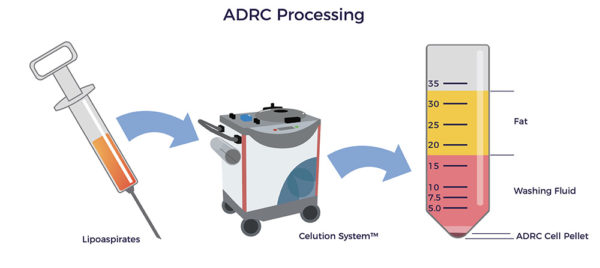 More than 40 countries have approved Celution for clinical use, including the United Kingdom, the European Union, Japan, South Korea, and New Zealand. Additionally, FDA has approved Celution for nine clinical trials.
More than 40 countries have approved Celution for clinical use, including the United Kingdom, the European Union, Japan, South Korea, and New Zealand. Additionally, FDA has approved Celution for nine clinical trials.
Since 2007 approvals for Celution in Europe and Japan, no reported cell-related adverse events have been reported in reported trials, studies, and clinical use. [32]
[2] Tracey K The inflammatory reflex Nature Vol 420 19/26 December 2002
[3] Blaszkiewicz et al. The involvement of neuroimmune cells in adipose innervation Mol Med (2020) 26:126
[4] Simora N et al. The Role of the Immune System in Metabolic Health and Disease Cell Metabolism 25, March 7, 2017
[5] Amiya E MD PHD et al The Relationship between Vascular Function and the Autonomic Nervous System Ann Vasc Dis Vol. 7, No. 2; 2014; pp 109–119 Online Month May 16, 2014
[6] Okita A, Murakami Y, Shimokawa S, et al. Changes of serum inflammatory molecules and their relationships with visual function in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2020;61(11):30.
[7] Vinik A I The conductor of the autonomic orchestra June2012 Volume3 Article71 13
[8] Limoli P G et al. Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa Antioxidants 2020, 9, 983
[9] Sorrentino FS, Bonifazzi C, Paolo P The Role of the Endothelin System in the Vascular Dysregulation Involved in Retinitis Pigmentosa Journal of Ophthalmology Volume 2015, Article ID 405234
[10] Murakami, Y et al, (2018), C-Reactive protein and progression of vision loss in retinitis pigmentosa. Acta Ophthalmol, 96: e174-e179.
[11] Wang S, Lu B, Girman S, Duan J, McFarland T, et al. (2010) Non-Invasive Stem Cell Therapy in a Rat Model for Retinal Degeneration and Vascular Pathology. PLoS ONE 5(2): e9200.
[12] Zhao T et al. Intravenous Infusion of Umbilical Cord Mesenchymal StemCells Maintains and Partially Improves Visual Function in Patients with Advanced Retinitis Pigmentosa STEM CELLS AND DEVELOPMENT Volume 29, Number 16, 2020
[13] Grant ZL et al. Blocking endothelial apoptosis revascularizes the retina in a model of ischemic retinopathy J Clin Invest. 2020;130(8):4235-4251
[14] Lang M et al. Vascular dysfunction in retinitis pigmentosa Acta Ophthalmol. 2019: 97: 660–664
[15] Zhao T, Lie H, Wang F, Liu Y, Meng X, Yin Z and Li S (2021) Comparative Study of a Modified Sub-Tenon’s Capsule Injection of Triamcinolone Acetonide and the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Retinitis Pigmentosa Combined With Macular Edema. Front. Pharmacol. 12:694225.
[16] Hirose Y et al. Comparison of trophic factors secreted from human adipose-derived stromal vascular fraction with those from adipose-derived stromal/stem cells in the same individuals Cytotherapy, 2018; 20: 589–591
[17] S Kesten and JK Fraser Autologous Adipose Derived Regenerative Cells: A Platform for Therapeutic Applications Advanced Wound Healing Surgical Technology International XXIX
[18] Visoso F. J. et al Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities Int. J. Mol. Sci. 2019, 20, 3738
[19] Caplan A I Mesenchymal Stem Cells: Time to Change the Name! STEM CELLS TRANSLATIONALMEDICINE 2017; 6:1445–1451
[20] A Caplan PhD MSCs: The Sentinel and Safe-Guards of Injury J. Cell. Physiol. 231: 1413–1416, 2016.
[21] Razavi, Shahnaz et al. “Neurotrophic Factors and Their Effects in the Treatment of Multiple Sclerosis.” Advanced Biomedical Research 4 (2015): 53. PMC. Web. 28 Sept. 2018.
[22] J. K. Huang et al Myelin Regeneration in Multiple Sclerosis: Targeting. Endogenous Stem Cells., The American Society for Experimental NeuroTherapeutics, Inc. 2011
[23] T Lopatina et al. (2011) Adipose-Derived Stem Cells Stimulate Regeneration of Peripheral Nerves: BDNF Secreted by These Cells Promotes Nerve Healing and Axon Growth De Novo. PLoS ONE 6(3): e178991
[24] S. Seigo et al, Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration, Journal of Orthopaedic Science, Volume 18, Issue 1,2013, Pages 145-151
[25] Xu et al Brain-derived neurotrophic factor reduces inflammation and hippocampal apoptosis in experimental Streptococcus pneumoniae meningitis Journal of Neuroinflammation (2017) 14:156
[26] Blaszkiewicz, M., Wood, E., Koizar, S. et al. The involvement of neuroimmune cells in adipose innervation. Mol Med 26, 126 (2020)
[27] Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. 2012; 763:70-84.
[28] L. Liu et al From Blood to the Brain: Can Systemically Transplanted Mesenchymal Stem Cells Cross the Blood-Brain Barrier? Stem Cells International Volume 2013, Article ID 435093
[29] A. Laroni et al Mesenchymal stem cells for the treatment of neurological diseases: Immunoregulation beyond neuroprotection Immunology Letters 168 (2015) 183-190
[30] M. Absthina et al Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI eLife 2017;6: e 29738
[31] Perin EC and Willerson JT Buying New Soul J Am Coll Cardiol. 2012;60(21):2250-2251
