Breaking Eroom’s Law
Breaking Eroom’s Law
“In solving a problem of this sort, the grand thing is to be able to reason backwards. That is a very useful accomplishment, and a very easy one, but people do not practise it much. In the every-day affairs of life it is more useful to reason forwards, and so the other comes to be neglected.” Sherlock Holmes, A Study in Scarlet
Breaking Eroom’s Law
Between October 2014 and July 2025, a group of ~500 Ambrose Cell Therapy patients (including our predecessor group) broke the law, “Eroom’s Law.” [1] More on Eroom in a moment. First, what does Sherlock Holmes and backward reasoning have to do with proposing exponential improvements in safety, effectiveness, and the cost of healthcare?
Contrary to conventional and AI-driven drug development, we employed Sherlockian backward-reasoning to develop an adipose-derived regenerative cell (ADRC) platform therapy for rare genetic disorders. Counter-intuitively, our predecessor group pioneered our safe, experimental program with prevalent complex diseases such as congestive heart failure (CHF).
“Before my stem cell therapy in October 2014, I was told to prepare for hospice. I was only 71, but I had been admitting to the hospital every three months with heart failure.
Since my stem cells, I have only had one cardiac-related ER visit – for a brief Afib episode in 2024. Stem cells (ADRCs) saved my life,” extolled Patsy on her 81st birthday. – Patsy- Class IV congestive heart failure[2]

Compared to the $1.3 million lifetime cost of a heart transplant or the $130,000 per year cost of Class IV heart failure care, Patsy’s dramatic improvement from a single ADRC-based therapy signified that a new era of healthcare had begun.
Ambrose Patient-Reported Outcomes demonstrate a consistent pattern of sustained benefits while significantly reducing the cost of healthcare.
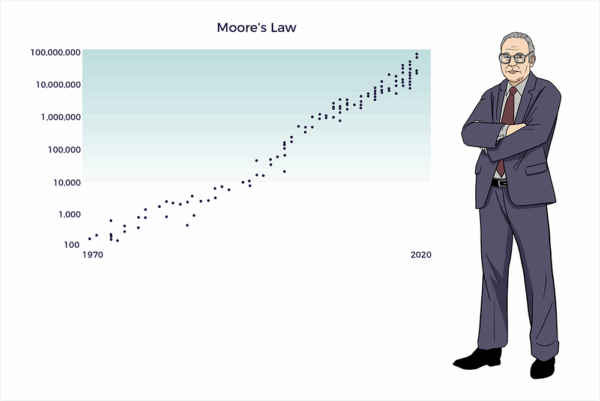
Moore’s Law
In 1965, Gordon Moore, Co-Founder of Intel Corp, observed that the number of transistors in a computer chip had doubled yearly. Moore’s Law evolved into the principle that the speed and capability of computers would double every two years.
With AI, computing power continues its exponential growth. As a result, the Information Age evolved backward from mainframes to minis to PCs to handhelds to wearables and implantables.
Eroom’s Law
Making a mockery of Big Pharma, Dr. Jack Scannell and colleagues spelled “Moore’s” backward, i.e., Eroom’s Law. (Nature Review Drug Discovery 2012). Here, they point out that drug discovery efficiency and efficacy experienced an exponential decades-long decline.
“Yet the number of new drugs approved per billion US dollars spent on R&D has halved roughly every 9 years since 1950, falling around 80-fold in inflation-adjusted terms.”[3]
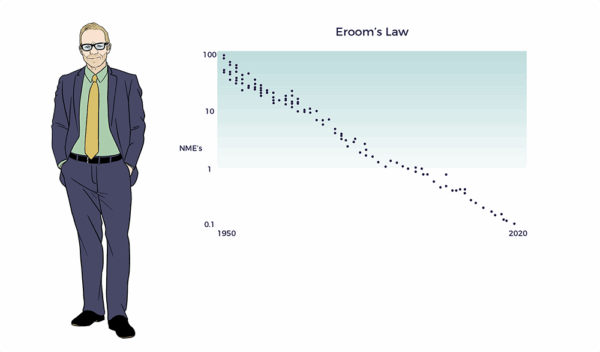
An 80x decline represents a 98.75% decrease in cost-risk-benefit.
Reversing Eroom’s Law
Following the example set by Moore’s Law, we reviewed Ambrose’s follow-up data on dozens of patients who had suffered from diverse chronic conditions. We concluded that Eroom’s law is far from set in stone.
Rhonda’s three-year outcome embodies the potential for an Ambrose’s Law for healthcare.
“When I was 60, I had a major cerebral aneurysm. I spent two months in the ICU and was in and out of the hospital for the next eight months.
Before Ambrose, I took gabapentin, pain meds, and was calling 911 nearly every week with chronic back pain and sciatica. I took five blood pressure meds and used two inhalers for COPD-induced asthma. I watched TV with the volume at 72, not because I couldn’t hear it, but because I couldn’t remember what was being said.
Since (Ambrose) stem cells, I walk, garden, cook for my family and friends on holidays, and take care of my grandchildren. My cardiologist says I am a medical miracle, and my neurologist tested me and said my memory is” 99%”. Thank God for stem cells.”
Rhonda – 70 years old.

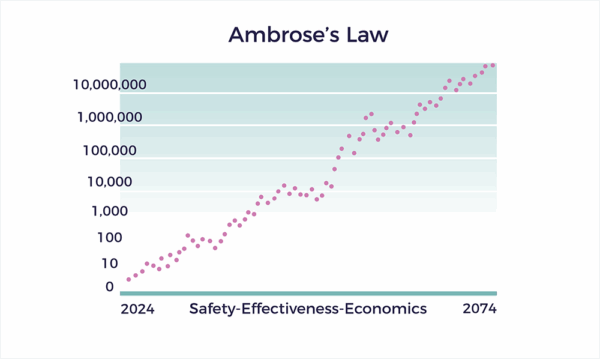
Ambrose’s Law
Ambrose’s Law states that the Ambrose Master Protocol spurs exponential growth in safety, effectiveness, and economic value for patients living with complex chronic conditions.
Ambrose’s Law requires backward reasoning from different directions. To accomplish exponential growth in healthcare outcomes and economics, we:
- provide Ambrose Cell Therapy as a service instead of putting a drug in a vial
- process ADRCs at the point of care instead of requiring a manufacturing plant
- price our one-time cell therapy priced at <10% of the lifetime cost of conventional care and less than 5% of the all-in cost of gene therapies
- target a 90% positive response rate with durable benefits and no cell-related adverse events.
Pharma’s Immutable Irrelevance
Ten years after Scannell’s scathing criticism of drug development, J Howick et al. (2022) doubled down on the risk and lack of reward of commonly prescribed drugs. “In this large sample of 1567 interventions studied within Cochrane reviews, only 5.6% were supported by high-quality evidence. Potential harms of healthcare interventions were measured more rarely than benefits.” [4]
Unimaginably, in 2025, Deloitte observed that the cost of a new drug approval increased from $1.3 billion to $2.2 billion since Scannell published Eroom’s Law.[5] Between Scannell, Howick, and Deloitte’s findings, this brings the fall to around 160 times.
In other words, since 1950, the net value of new drugs, biologics, and cell and gene therapies has approximated a bagel hole.
Rare Reasoning
Our backward reasoning leads to seeking FDA approval for an ADRC-based therapy for a rare genetic disorder and expanding from there.
- Beginning in 2007, prestigious medical research centers, including the University of Tokyo, Erasmus University, Texas Heart Institute, and Cedars-Sinai, conducted 71 clinical trials or experimental cases in which they treated 1,471 patients with Celution®-processed ADRCs.

- Over 10 years, Ambrose (and our predecessor group) treated over 500 no-option patients with heart failure, dementia, Parkinson’s disease, Crohn’s disease, or other prevalent conditions.
- Over that same 10-year period, Ambrose provided ~40 patients living with rare diseases the right to try their ADRCs—approximately 90% of those patients reported life-changing benefits despite their genetic mutations.[6]
- Ehlers-Danlos Syndrome =25
- Nail-Patella Syndrome =1
- Marfan Syndrome =3
- Rett Syndrome =1
- Dysgenesis of the Corpus Callosum =1
- Primary hemochromatosis =1
- Sickle cell thalassemia =1
- Rasmussen’s encephalitis with refractory seizures =1
- Synovial chondromatosis =1
- Post-cortical atrophy Alzheimer’s =1
- Multiple system atrophy – C =2
- Frontal temporal dementia =2
Our bold approach defies the forward-reasoning drug discovery dogma. Instead, we follow those who developed In Vitro Fertilization (IVF) for infertility and bone marrow transplants for blood cancers outside of formal FDA-approved trials.
Cracking the Genetic Disorder Code
Just as Holmes deciphered a family riddle in “The Musgrave Ritual” (1893),
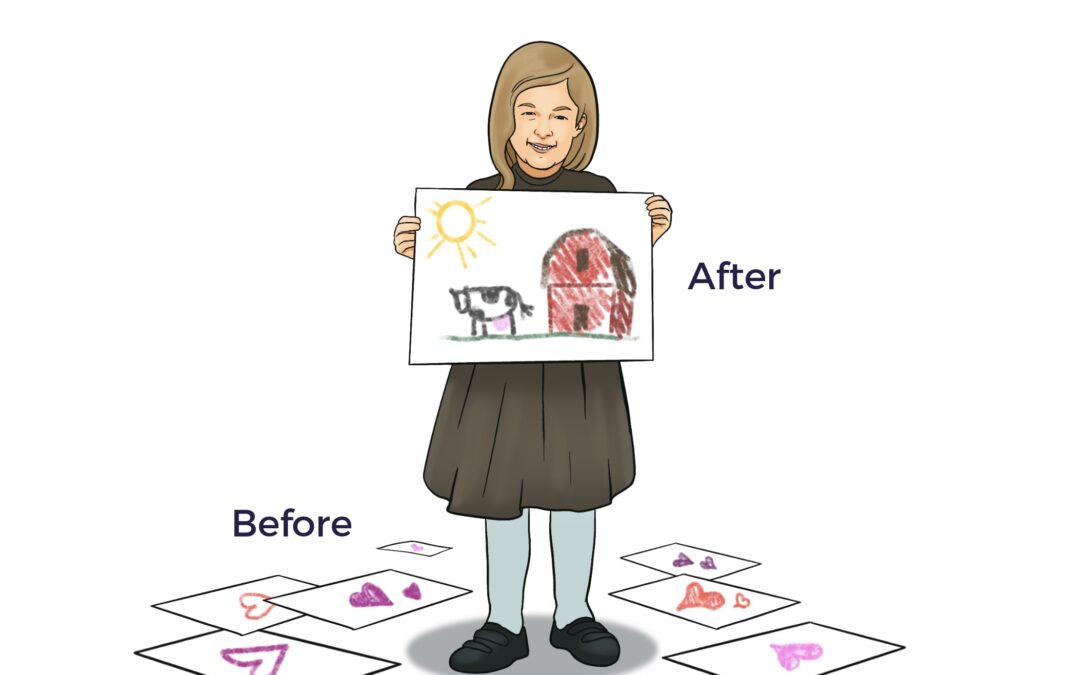
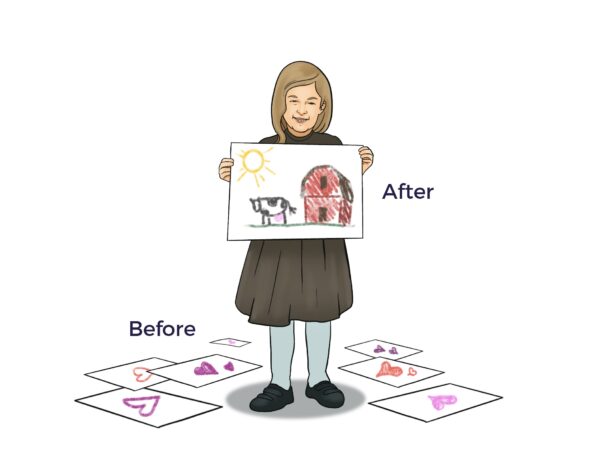
Ambrose cracked the cellular codes for Emilie, who lived with sickle cell thalassemia and Marfan Syndrome, a connective tissue disease.
 Emilie’s Reversal
Emilie’s Reversal
After suffering from severe sickle cell thalassemia for seven years, in August 2023, Emilie flew from London to Los Angeles to exercise her Right to Try Ambrose Cell Therapy. Since Emilie’s one-time Ambrose treatment, she has not had a single severe sickle crisis.[7]
“Before Ambrose, I admitted to the A&E (accident and emergency department) with a severe sickle cell crisis nearly every week. I went from being very sick, then after my (Ambrose) treatment, my body went into healing mode. I realized a couple of days ago that the healing phase feels like it’s over.
I feel different. It’s more stable. It’s moved: next stage, next stage, and next stage.
Lili, my oldest daughter, treated me to a spa day for my (42nd) birthday. I swam in the pool for 40 minutes.
I couldn’t do that before because the cold water caused pain. I slept great. I had never gone swimming with my daughter. She’s 22.
It really, really, really is incredible. Having had this realization, I feel better spiritually.”

Saving Money While Saving a Life
In just the past two years, Emilie’s ADRCs would have saved the US healthcare system $135,000.
“Compared to matched controls, patients with SCD with recurrent VOCs (vaso-occlusive crises)* had significantly higher annual ($67,282 vs. $4134) and lifetime ($3.8 million vs. $229,000 over 50 years) healthcare costs, highlighting the need for cost-effective therapies that reduce clinical complications in this patient population“. (C Udeze et al. 2023)[8]
* A vaso-occlusive crisis (VOC), or sickle cell crisis, occurs when sickled red blood cells block small blood vessels. The restricted blood flow to tissues and organs causes severe pain.
However, that understates her long-term benefits and the cost savings to the system by an unfathomable amount. In February of 2023, Emilie had gotten her affairs in order and told her three children goodbye.
She was only 39 at the time, but Emilie didn’t believe she would make it to her 40th birthday, much less 50, the lifespan of the average person living with severe sickle cell disease.
Eroome’s Law Strikes Gene Therapy
In comparison to Emilie’s one-time cost of ~$50,000, the two FDA-approved sickle cell gene therapies, Casgevy (Vertex, Inc.) and Lyfgenia (blue bird bio, Inc.), have wholesale costs of $2,100,000 and $3,000,000 (with a guarantee), respectively. But the costs don’t end there—medical visits, the hospital costs for preconditioning with chemo and the treatment, and then the post-chemo related side effects, including infertility, pile on $100s of thousands more.

Sparse patient uptake led to blue bird bio, Inc. being “taken under” for $30 million by Carlyle. Investors once valued the Company at over $10 billion. Eroom’s Law strikes again.
The Connective Tissue Issue
Ambrose’s Law took hold when an increasing number of patients living with rare connective tissue diseases accessed their ADRCs under the Right to Try Act of 2017. Specifically, Ambrose patients living with Ehlers-Danlos Syndrome (EDS), Nail-Patella Syndrome (NPS), and Marfan Syndrome declared exponential improvements while lowering their ongoing costs of care, just as the doubling of transistors in a microchip had done for computing, thanks to Gordon Moore.
Most surprisingly, patients living with ultra-rare EDS variants – those observed in less than one in a million people – super-responded. From the odds angle, ADRCs reversed the odds of little or no response from one in a million to approximately nine-in-ten in ten reporting life-changing benefits.
“The most important thing is that I truly am better; whatever was causing my physical decline is no longer there. It is amazing.
Thank you for all your help and for all you are doing to get this regenerative therapy program into broad use.” Anna – 60 years old, Arthrochalasia EDS
“You’re bringing healing solutions to people who felt helpless and hopeless. You’re helping them to live outside of a body-centric existence. I’m so proud of you.” – Sally – 48 years old, Spondylodysplastic type I EDS
“I was an invalid. I am slowly getting better. I am sleeping 6-8 hours at a time, instead of waking up every two hours in pain, I am off all meds and able to walk with a cane.” – Melissa, 80 years Old, Ehlers-Danlos Syndrome, Dermatosparaxis Type
The remarkable Ambrose patient-reported outcomes of ~25 patients living with Ehlers-Danlos Syndrome, Marfan Syndrome, Nail Patella Syndrome, and ~10 other rare genetic disorders inspired our audacious vision.[9] (For more information, A Golden Era of ADRC-Therapy for Rare Connective Tissue Disorders is available upon request. contact@ambrosecelltherapy.com )
Moore’s Law Shortfall
While high-tech moguls like Jeff Bezos, Mark Zuckerberg, and Sergey Brin have leveraged Moore’s Law to flaunt their superyachts, Gen X, Millennials, Gen Z, and Gen Y come down with more chronic diseases at younger ages with each succeeding generation. Thus, Eroom’s Law impacts all of our lives.
Backwards Business Model Reasoning
“The opposite of a good idea is another good idea.” Rory Sutherland, Vice-Chairman, Ogilvy UK

Profits before patients has been a good idea for big pharma shareholders. In 2024, Eli Lilly, AbbVie, Johnson & Johnson, and seven other mega-cap drug companies reported over $100 billion in profits with near-zero paid in US taxes.
In comparison, patients pay an average of 25% of their income in federal, state, and local taxes. To add insult to injury, our tax dollars fund the payors, i.e., Medicare, Medicaid, and private insurance companies. Then the payors overpay big pharma for overpriced meds that don’t work very well.

Patients Before Profits
Our opposite good idea puts patients before profits. We propose pricing our one-time cell therapy at less than 5% of the lifetime costs of rare disease drugs and gene therapies. To be clear, Ambrose aims to make a profit, but in exchange for creating value for patients, their families, and society.
Moore’s Law created trillions of dollars of shareholder value, but Mr. Moore’s dream that computing power could exponentially change the world for the better fell short. Boldly, we postulate that Ambrose’s Law will catalyze a safe and effective healthcare ecosystem, vanquish Eroom’s Law, and change the world in tribute to Gordon Moore.

[1] Based on patient-reported outcomes, physician, and family reports.
[2] Patient Quotes are edited for clarity and brevity. Pseudonyms are used for patient privacy when requested.
[3] Scannell et al. Diagnosing the decline in pharmaceutical R&D efficiency Nature Review/Drug Discovery Vol 11 March 2012 191
[4] Howick, Jeremy et al. “Most healthcare interventions tested in Cochrane Reviews are not effective according to high quality evidence: a systematic review and meta-analysis.” Journal of clinical epidemiology vol. 148 (2022): 160-169.
[5] Terry C & Dondarski K. Deloitte Be brave, Be bold Measuring the return from pharmaceutical innovation 15th edition March 2025
[6] Based on patient-reported data believe to be reliable
[7] A Golden Era of ADRC-Based Therapy for Genetic Disorders – available upon request contact@ambrosecelltherapy.com
[8] Udeze, Chuka et al. “Economic and Clinical Burden of Managing Sickle Cell Disease with Recurrent Vaso-Occlusive Crises in the United States.” Advances in therapy vol. 40,8 (2023): 3543-3558.
[9] Based on patient and caregiver-reported data believed to be reliable



 Yet pharma and biotech focus on their unvaried one drug, one disease (or symptom) model. ASD’s diversity of causes and range of symptoms makes that approach unworkable. Additionally, the prescribed drugs often come with black box warnings, a multitude of potential side effects, and unpredictable interactions.
Yet pharma and biotech focus on their unvaried one drug, one disease (or symptom) model. ASD’s diversity of causes and range of symptoms makes that approach unworkable. Additionally, the prescribed drugs often come with black box warnings, a multitude of potential side effects, and unpredictable interactions. 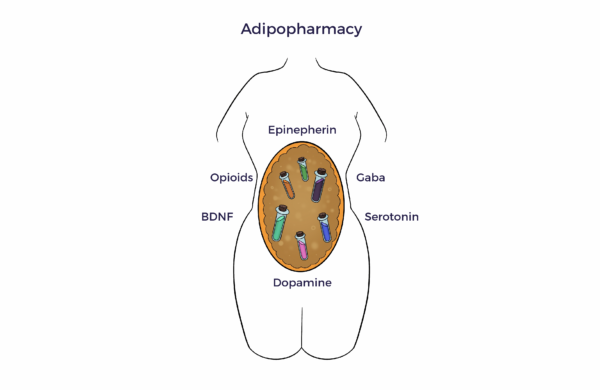
 “In my eyes and ears, the organ is the King of Instruments.”
“In my eyes and ears, the organ is the King of Instruments.”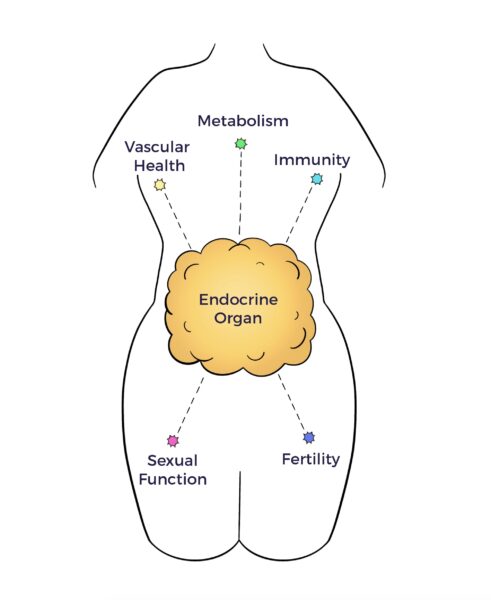
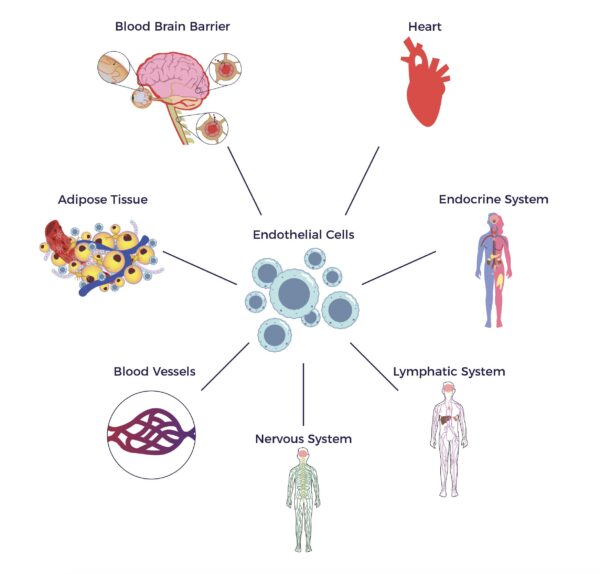
 In 2001, UCLA and University of Pittsburgh researchers unveiled a pool of multipotent cells in adipose tissue.
In 2001, UCLA and University of Pittsburgh researchers unveiled a pool of multipotent cells in adipose tissue.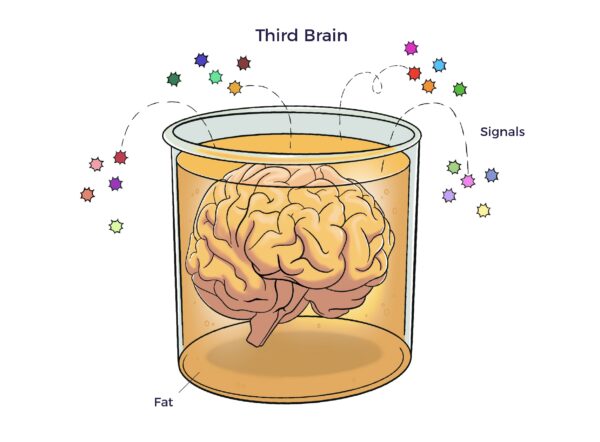 The adipose tissue as a third brain (Chaldakov et al. 2009) connects the adipose tissue secretome with neurochemical, ANS, and multisystem harmony.
The adipose tissue as a third brain (Chaldakov et al. 2009) connects the adipose tissue secretome with neurochemical, ANS, and multisystem harmony.


 In Oct 2021, Mary Grace, a 58-year-old adventurer, retraumatized her left knee trekking the Himalayas. Until then, she had surmounted a hospitalizing concussion, sports injuries, constant pain, brain fog, and chronic fatigue to scale new heights. But this incident was different – an air ambulance brought her down the mountain.
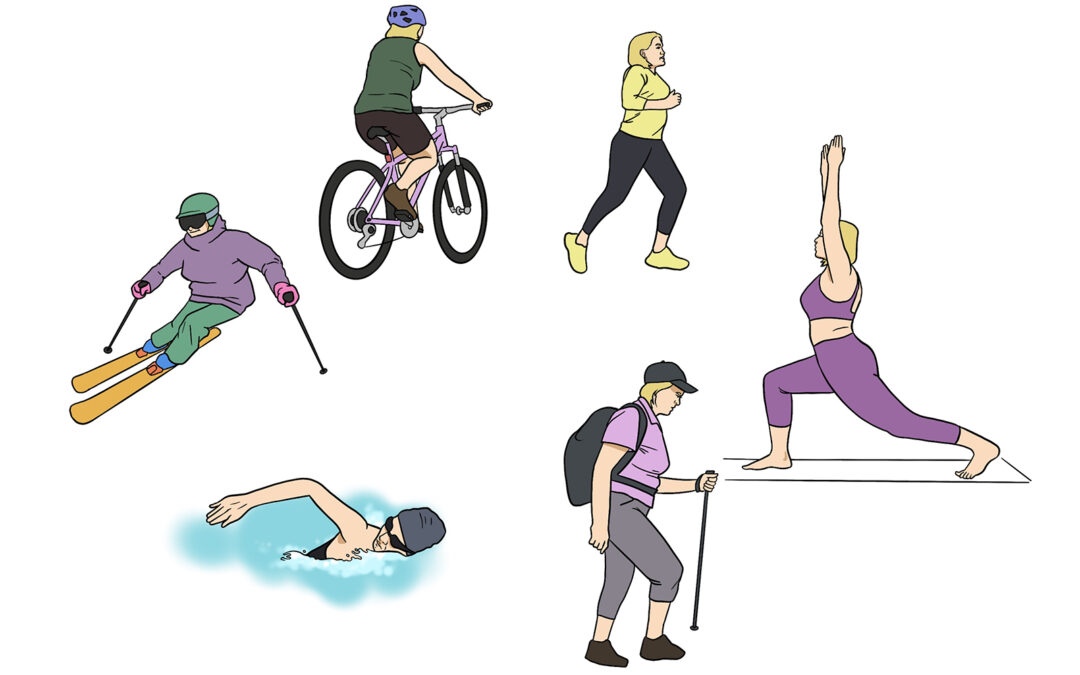
In Oct 2021, Mary Grace, a 58-year-old adventurer, retraumatized her left knee trekking the Himalayas. Until then, she had surmounted a hospitalizing concussion, sports injuries, constant pain, brain fog, and chronic fatigue to scale new heights. But this incident was different – an air ambulance brought her down the mountain. Over the 24 months following her single Ambrose treatment, Mary Grace resumed hiking, skiing, and open-water swimming in Mexico – against the tide, no less. She took up windsurfing alongside whales, too.
Over the 24 months following her single Ambrose treatment, Mary Grace resumed hiking, skiing, and open-water swimming in Mexico – against the tide, no less. She took up windsurfing alongside whales, too.
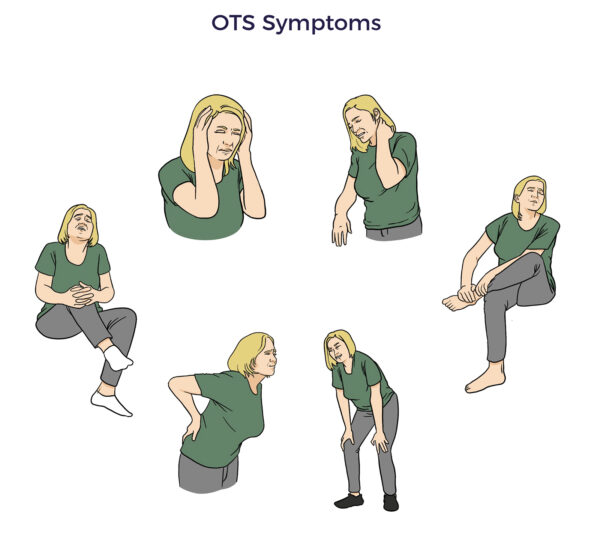 Mary Grace should have been a pillar of health as she approached her sixties, a pivotal point in healthspan. However, her love of demanding sports resulted in overtraining syndrome (OTS).
Mary Grace should have been a pillar of health as she approached her sixties, a pivotal point in healthspan. However, her love of demanding sports resulted in overtraining syndrome (OTS).
 Vagus Nerve
Vagus Nerve In contrast to Mary Grace’s near pain-free turnaround, Tiger Woods epitomizes the risks of excessive training and relying on surgeries, followed by medications, to manage his pain and depression.
In contrast to Mary Grace’s near pain-free turnaround, Tiger Woods epitomizes the risks of excessive training and relying on surgeries, followed by medications, to manage his pain and depression. While Mary Grace is not a world-class competitive athlete, the comparison holds. Few women have adventured to so many places in so many ways as her.
While Mary Grace is not a world-class competitive athlete, the comparison holds. Few women have adventured to so many places in so many ways as her.
 “People often ask if stem cells work. Of course, they work. We are all walking stem cell products – the sperm, and the egg,” the revered James Willerson, MD, Ph.D. said in a talk in 2011.
“People often ask if stem cells work. Of course, they work. We are all walking stem cell products – the sperm, and the egg,” the revered James Willerson, MD, Ph.D. said in a talk in 2011.
 Sadly, as a chiropractor’s patient with long-Covid and congestive heart failure related, “I tried umbilical cord stem cells. They didn’t work.” There were no live stem cells in the product with which he was treated or other commercially available perinatal vials, studies revealed:
Sadly, as a chiropractor’s patient with long-Covid and congestive heart failure related, “I tried umbilical cord stem cells. They didn’t work.” There were no live stem cells in the product with which he was treated or other commercially available perinatal vials, studies revealed: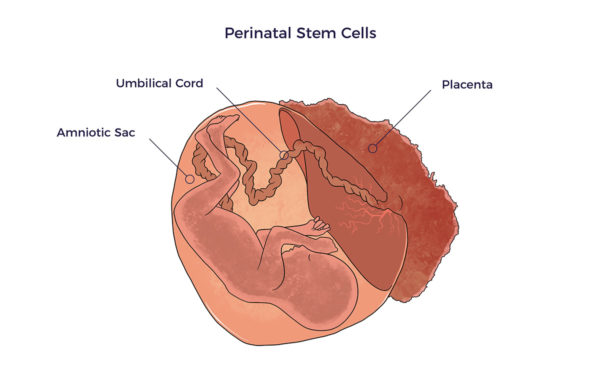 Perinatal refers to just before or shortly after birth. Thus, perinatal stem cells come from birth tissues or fluids, i.e., umbilical cord blood and tissue, placental blood and tissue, and amniotic tissue and fluid.
Perinatal refers to just before or shortly after birth. Thus, perinatal stem cells come from birth tissues or fluids, i.e., umbilical cord blood and tissue, placental blood and tissue, and amniotic tissue and fluid. Cells release tiny sacs or vesicles called exosomes. Like carrier pigeons, exosomes carry messages to nearby cells.
Cells release tiny sacs or vesicles called exosomes. Like carrier pigeons, exosomes carry messages to nearby cells.
 A doctor wanting to participate in the stem cell wild, wild west asked a lawyer specializing in stem cell regulatory affairs:
A doctor wanting to participate in the stem cell wild, wild west asked a lawyer specializing in stem cell regulatory affairs: Contrary to the FDA’s warning letters, a NextHealth patient coordinator insisted that Vitti’s and Organicell’s products are FDA-approved and that the manufacturers certify all lots are contaminant-free. Where did she get that idea?
Contrary to the FDA’s warning letters, a NextHealth patient coordinator insisted that Vitti’s and Organicell’s products are FDA-approved and that the manufacturers certify all lots are contaminant-free. Where did she get that idea? At the other end of the spectrum,
At the other end of the spectrum,  “Of course, they work. We are all walking stem cell products – the sperm, and the egg.” James Willerson, MD, Ph.D. Dr. Willerson went on to publish Buying New Soul (2012). Here he hypothesized that adipose tissue was the best source of adult stem cells.
“Of course, they work. We are all walking stem cell products – the sperm, and the egg.” James Willerson, MD, Ph.D. Dr. Willerson went on to publish Buying New Soul (2012). Here he hypothesized that adipose tissue was the best source of adult stem cells.





 Sam was a productive dentist, recreational golfer, proud parent, and happy husband. However, in 2013 his wife Flo, a retired physician, began a bold twenty-year battle to reverse his dementia, vascular disease, and pre-diabetes. In April 2020, Sam suffered a stroke and contracted Covid and pneumonia in the hospital. He remained there for two months.
Sam was a productive dentist, recreational golfer, proud parent, and happy husband. However, in 2013 his wife Flo, a retired physician, began a bold twenty-year battle to reverse his dementia, vascular disease, and pre-diabetes. In April 2020, Sam suffered a stroke and contracted Covid and pneumonia in the hospital. He remained there for two months. Further, the Greeks developed the first scientific considerations about blood. Back in Homer’s time (8th or 9th Century BC), they summarized four concepts that remain valid to day:
Further, the Greeks developed the first scientific considerations about blood. Back in Homer’s time (8th or 9th Century BC), they summarized four concepts that remain valid to day: In 1885, Rudolph Virchow coined “ischemia” to characterize the lack of blood flow in an organ or tissue. In plain words, ischemia means blood is not moving through your capillaries, blood vessels, veins, or arteries.
In 1885, Rudolph Virchow coined “ischemia” to characterize the lack of blood flow in an organ or tissue. In plain words, ischemia means blood is not moving through your capillaries, blood vessels, veins, or arteries. Sam’s long decline
Sam’s long decline Sam’s history epitomizes the complexity of age-related ill health:
Sam’s history epitomizes the complexity of age-related ill health: Flo also knew to steer clear of drug combinations, including anti-depressants, anti-psychotics, anti-seizure, and sleep meds (polypharmacy). Neurologists prescribe polypharmacy to patients with neurodegenerative diseases despite its well-documented contribution to the progression and severity of dementia. In other words, drug companies profit, and patients pay the price.
Flo also knew to steer clear of drug combinations, including anti-depressants, anti-psychotics, anti-seizure, and sleep meds (polypharmacy). Neurologists prescribe polypharmacy to patients with neurodegenerative diseases despite its well-documented contribution to the progression and severity of dementia. In other words, drug companies profit, and patients pay the price.  Flo believed in the body’s power to heal itself. That is why she was attracted to the RECODE protocol.
Flo believed in the body’s power to heal itself. That is why she was attracted to the RECODE protocol. Flo requested papers supporting potential benefits for Sam’s memory.
Flo requested papers supporting potential benefits for Sam’s memory.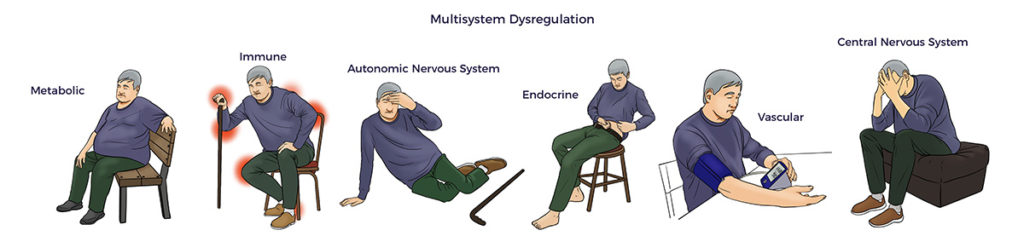
 Per the
Per the 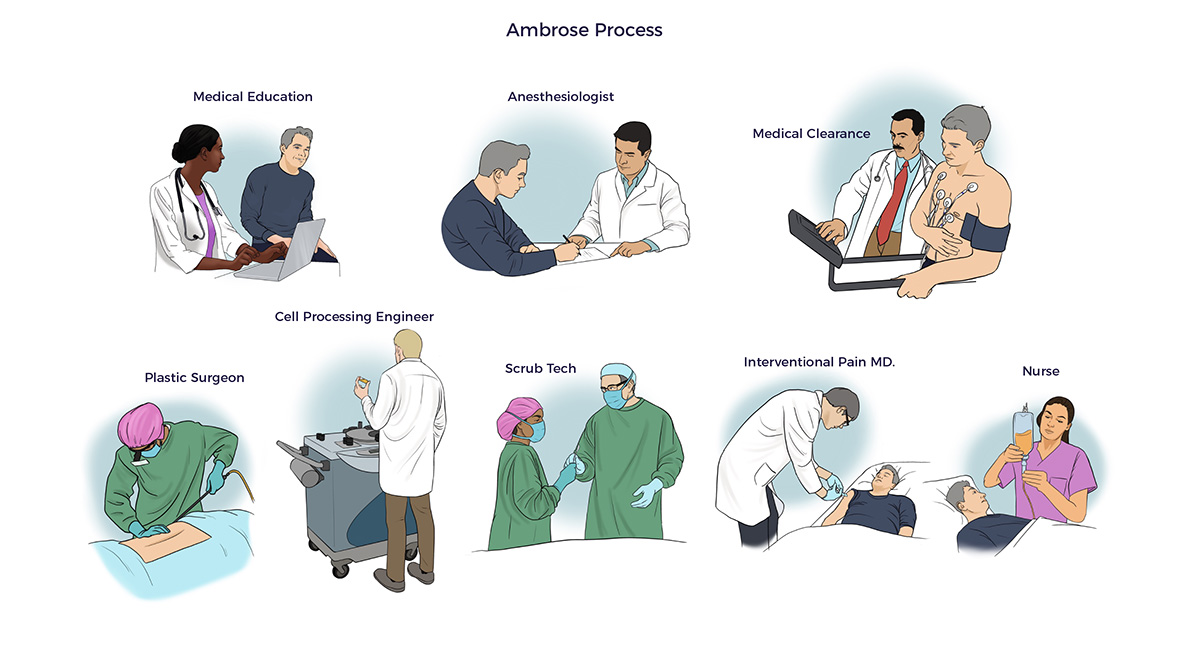
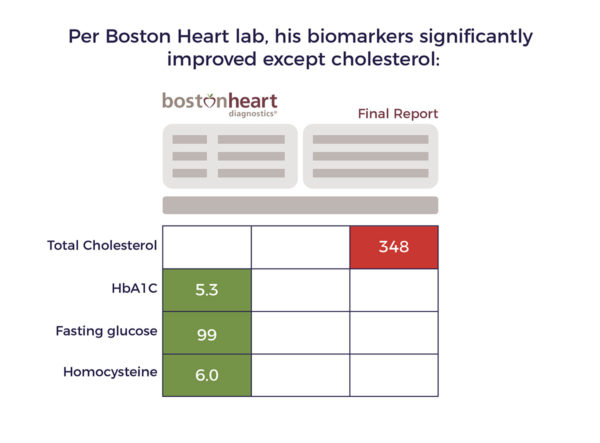 Consistent with the studies Ambrose provided to Flo, objective evidence indicates cell therapy lowered Sam’s risk of another catastrophic stroke or a heart attack.
Consistent with the studies Ambrose provided to Flo, objective evidence indicates cell therapy lowered Sam’s risk of another catastrophic stroke or a heart attack.

 RJ played baseball, football, snowboarding, and golf growing up.
RJ played baseball, football, snowboarding, and golf growing up.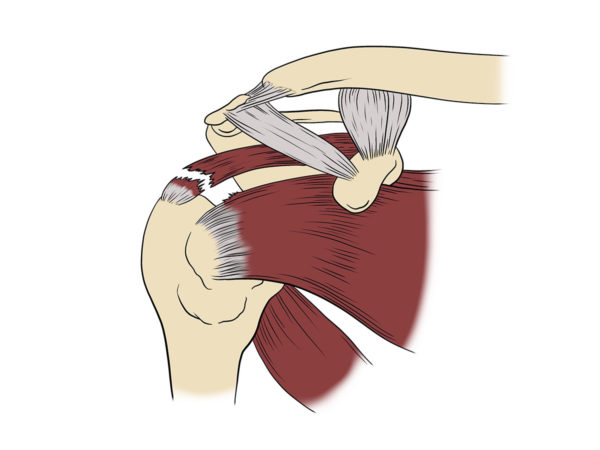 “Nothing showed up on my MRI and X-Ray, but I couldn’t move my arm. I went in for exploratory surgery. The surgeon found my rotator cuff and labrum were shredded.
“Nothing showed up on my MRI and X-Ray, but I couldn’t move my arm. I went in for exploratory surgery. The surgeon found my rotator cuff and labrum were shredded.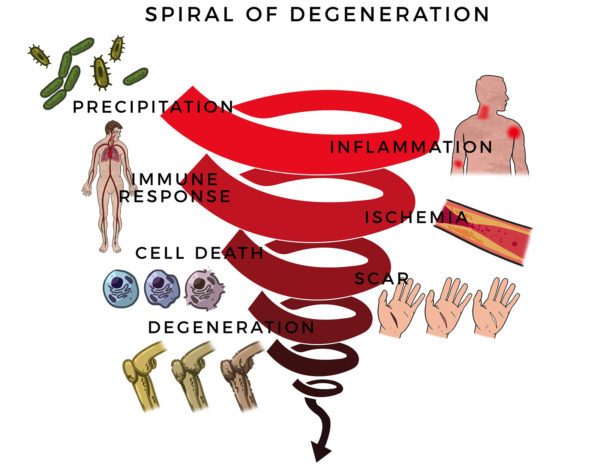 Here, RJ’s massive rotator cuff tear (MRCT) caused disability and pain. An MRCT precipitates a Spiral of Degeneration beginning with inflammation, abnormal immune response, and lack of blood flow. That leads to programmed cell death (apoptosis), scarring, and degeneration.
Here, RJ’s massive rotator cuff tear (MRCT) caused disability and pain. An MRCT precipitates a Spiral of Degeneration beginning with inflammation, abnormal immune response, and lack of blood flow. That leads to programmed cell death (apoptosis), scarring, and degeneration. More concerning, RJ experienced symptoms of multisystem dysregulation:
More concerning, RJ experienced symptoms of multisystem dysregulation:
 “I don’t feel depressed like I did before. I am optimistic and have more energy. I don’t wake up drenched with sweat and only need about eight hours of sleep now. I used to sleep from 12:00 am to 2:00 pm and take an hour nap too. Now I coach baseball and football 15 hours a day.”, RJ reported.
“I don’t feel depressed like I did before. I am optimistic and have more energy. I don’t wake up drenched with sweat and only need about eight hours of sleep now. I used to sleep from 12:00 am to 2:00 pm and take an hour nap too. Now I coach baseball and football 15 hours a day.”, RJ reported.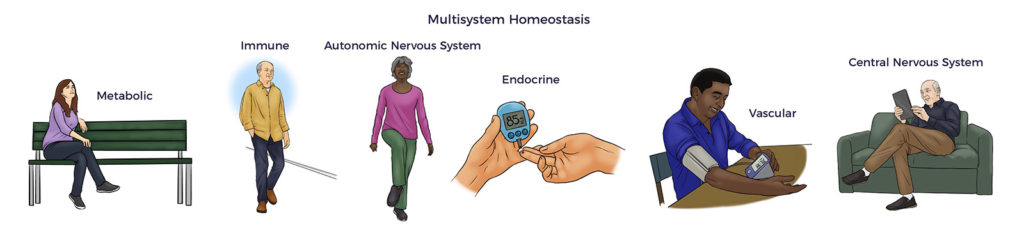
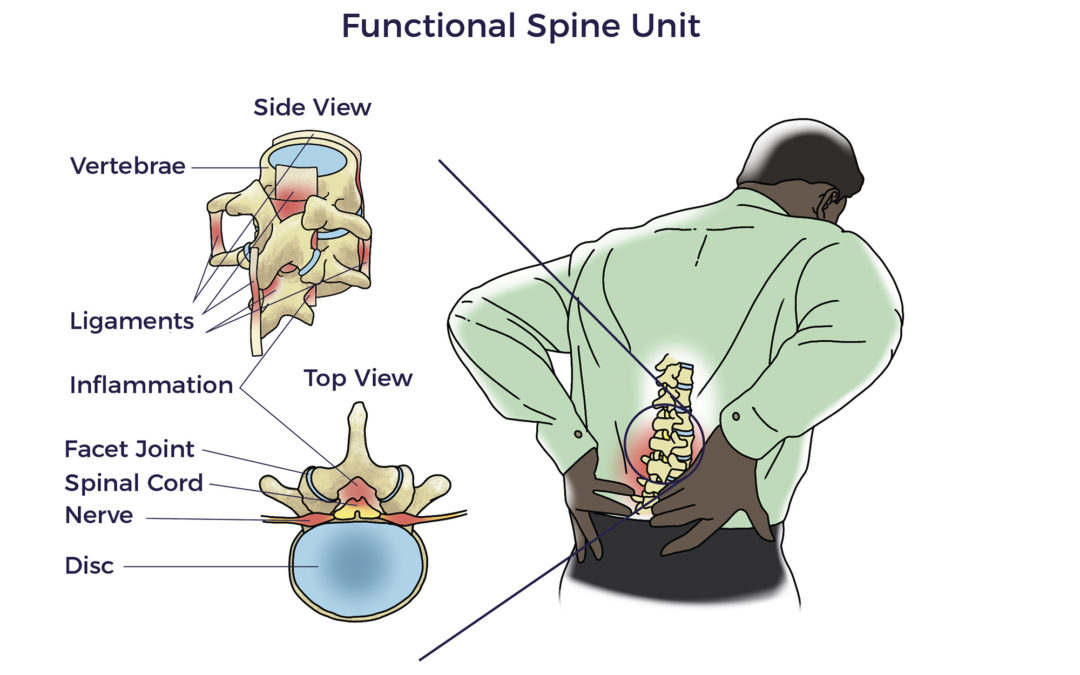
 Spine care has its origins in antiquity. The Edwin Smith surgical papyrus, an Egyptian document written in the 17th century BC, is the first known discussion of neck and back-related injuries. Hippocrates (4th century BC) experimented with traction or local pressure to correct spinal deformities. Aristotle also contributed to our current day understanding of the neck and spine.
Spine care has its origins in antiquity. The Edwin Smith surgical papyrus, an Egyptian document written in the 17th century BC, is the first known discussion of neck and back-related injuries. Hippocrates (4th century BC) experimented with traction or local pressure to correct spinal deformities. Aristotle also contributed to our current day understanding of the neck and spine.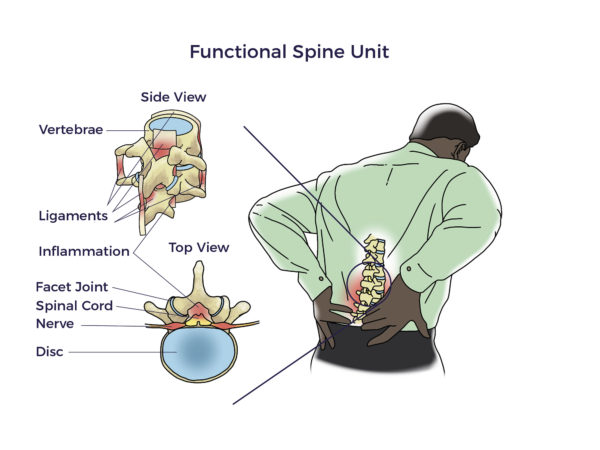 But there was still more to be discovered than the FSU. More recently, researchers began focusing on the vicious interplay involved with traumatic injuries, wear and tear, inflammation, and other diseases (co-morbidities). For example, patients with heart disease, diabetes, neurologic conditions, and autoimmune diseases have a higher prevalence of spine disorders than others without those conditions. In fact, Barb, Trish, Kathy, and Jeff each lived with other chronic debilitating conditions, including arthritis, hypermobility, kidney failure, and spinal cord injury, respectively.
But there was still more to be discovered than the FSU. More recently, researchers began focusing on the vicious interplay involved with traumatic injuries, wear and tear, inflammation, and other diseases (co-morbidities). For example, patients with heart disease, diabetes, neurologic conditions, and autoimmune diseases have a higher prevalence of spine disorders than others without those conditions. In fact, Barb, Trish, Kathy, and Jeff each lived with other chronic debilitating conditions, including arthritis, hypermobility, kidney failure, and spinal cord injury, respectively. 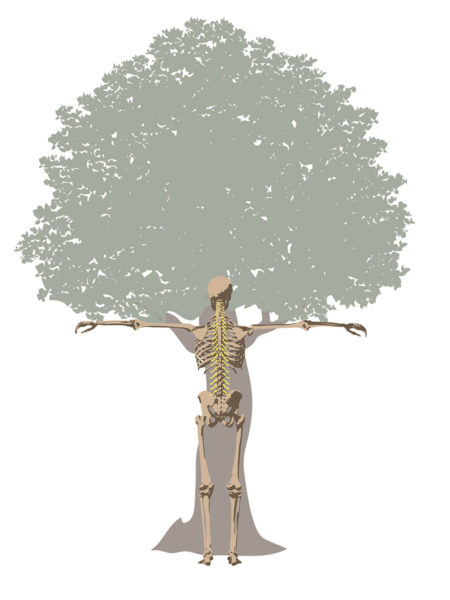 Circling back, in the early 1500s, Leonardo da Vinci took an unusual interest in tree anatomy. His Rule of Trees explained the balance between the trunk and branches of a tree. He counted the rings in tree trunks to determine “the nature of past seasons.”
Circling back, in the early 1500s, Leonardo da Vinci took an unusual interest in tree anatomy. His Rule of Trees explained the balance between the trunk and branches of a tree. He counted the rings in tree trunks to determine “the nature of past seasons.”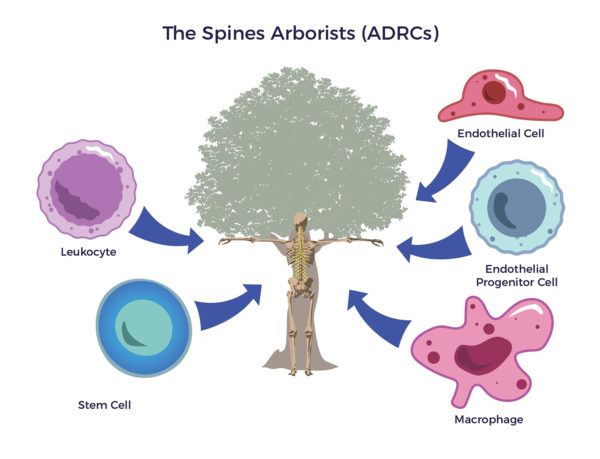 An arborist cultivates trees; tree removal is a last resort. The first thing that goes through their minds is how to save the tree. To do their jobs, arborists cultivate the whole tree. They use fertilizer, irrigation, and other regenerative tools to restore the tree’s trunk, limbs, and leaves.
An arborist cultivates trees; tree removal is a last resort. The first thing that goes through their minds is how to save the tree. To do their jobs, arborists cultivate the whole tree. They use fertilizer, irrigation, and other regenerative tools to restore the tree’s trunk, limbs, and leaves.
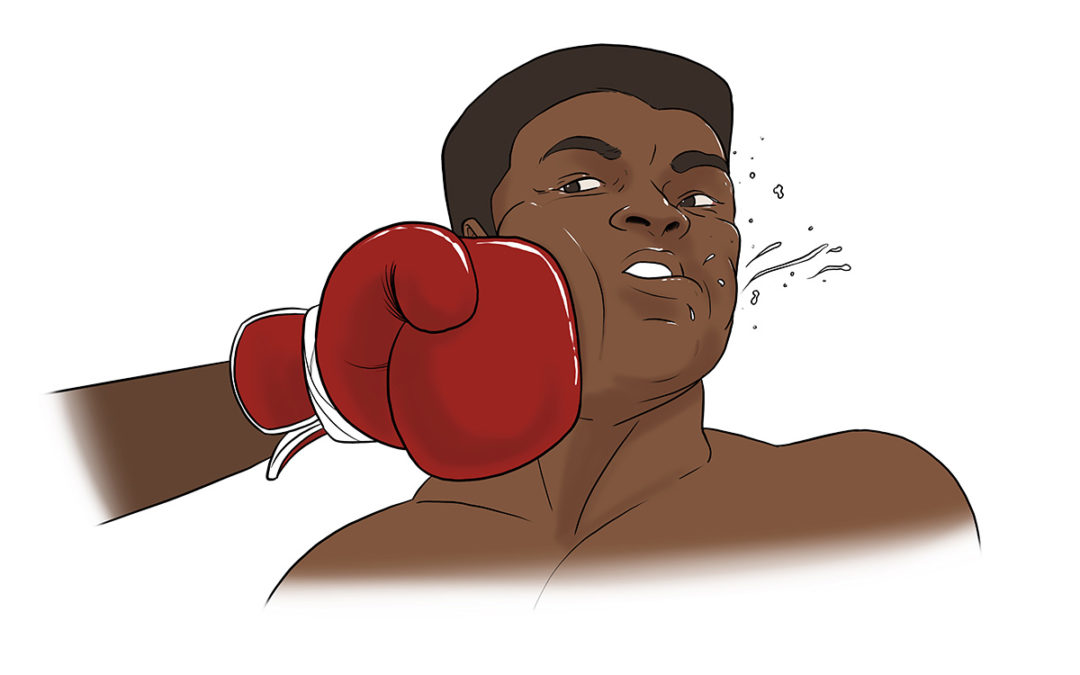
 Muhammed Ali’s 1960 Olympic Gold medal started his ascent to being “The Greatest,” but the countless blows to his head – which he welcomed to tire his opponent out – ended in a years-long failed fight to save his brain.
Muhammed Ali’s 1960 Olympic Gold medal started his ascent to being “The Greatest,” but the countless blows to his head – which he welcomed to tire his opponent out – ended in a years-long failed fight to save his brain.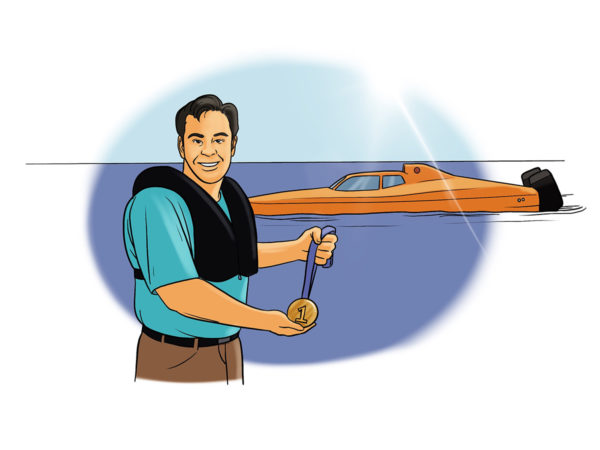 In 2000, Tim retired from offshore powerboat racing with four world speed records. He once hit upwards of 180 mph.
In 2000, Tim retired from offshore powerboat racing with four world speed records. He once hit upwards of 180 mph. Essential tremor is a nervous system (neurological) disorder that causes involuntary and rhythmic shaking. It can affect almost any part of your body, but the trembling occurs most often in your hands — especially when you do simple tasks, such as drinking from a glass, handwriting, or tying shoelaces.
Essential tremor is a nervous system (neurological) disorder that causes involuntary and rhythmic shaking. It can affect almost any part of your body, but the trembling occurs most often in your hands — especially when you do simple tasks, such as drinking from a glass, handwriting, or tying shoelaces.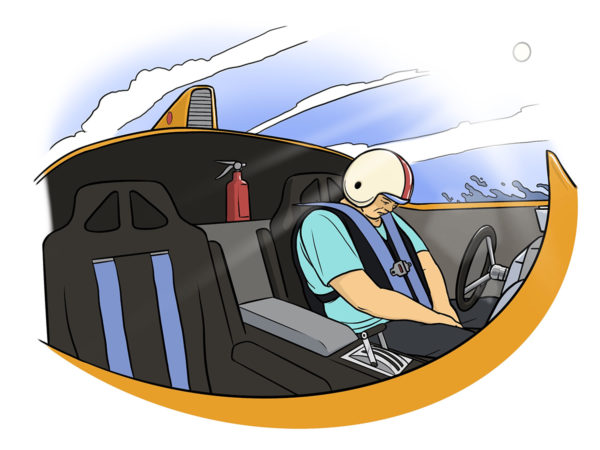 “I must have had 1,000 concussions while racing. I probably went unconscious 100 times behind the wheel from the G-forces,” Tim estimated.
“I must have had 1,000 concussions while racing. I probably went unconscious 100 times behind the wheel from the G-forces,” Tim estimated.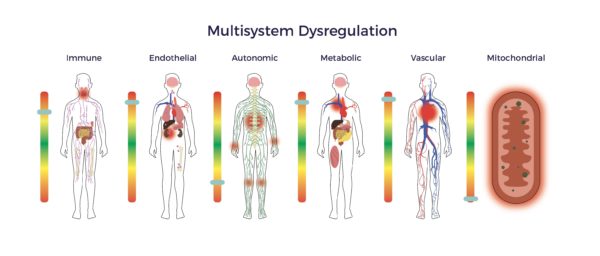
 To solve for this, doctors prescribe patients drugs for each ailment or symptom, e.g., Sinemet for Parkinson’s, Ambien for sleep, steroids for arthritis, opioids for pain, and so forth.
To solve for this, doctors prescribe patients drugs for each ailment or symptom, e.g., Sinemet for Parkinson’s, Ambien for sleep, steroids for arthritis, opioids for pain, and so forth.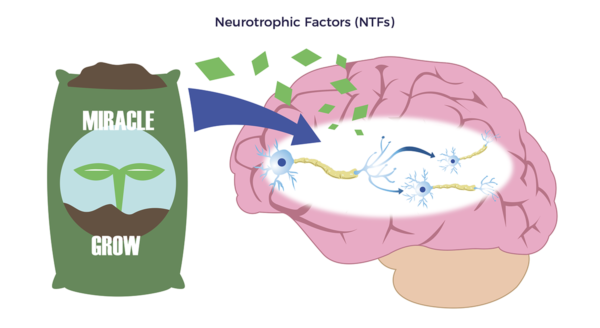
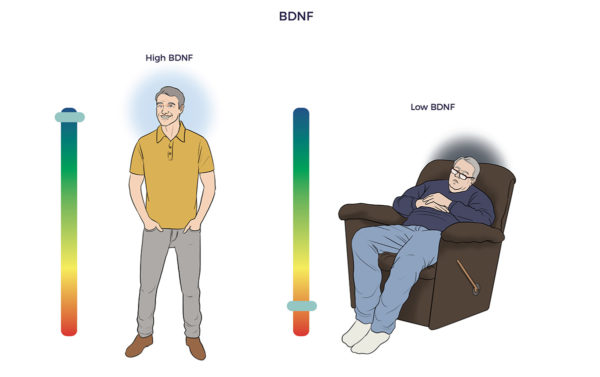 John Hopkins researchers developed a BDNF-blood test that could have predicted the severity of Ali’s head damage and how he would fare. Their study showed that patients with brain injuries have less than one-third of the BDNF as those with healthy brains.
John Hopkins researchers developed a BDNF-blood test that could have predicted the severity of Ali’s head damage and how he would fare. Their study showed that patients with brain injuries have less than one-third of the BDNF as those with healthy brains.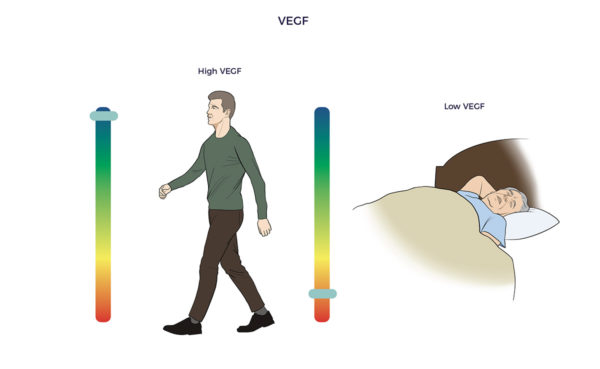 ADRCs also enrich the brain with vascular endothelial growth factor (VEGF). VEGF restores blood flow and reduces inflammation in withering tissues, blood vessels, and organs. One study found higher levels of VEGF in asymptomatic seniors who died with amyloid plaques compared to symptomatic Alzheimer’s patients.
ADRCs also enrich the brain with vascular endothelial growth factor (VEGF). VEGF restores blood flow and reduces inflammation in withering tissues, blood vessels, and organs. One study found higher levels of VEGF in asymptomatic seniors who died with amyloid plaques compared to symptomatic Alzheimer’s patients.