AMBROSE Cell Therapy for Neurodegenerative Diseases
Through decades of research, our understanding of neurodegenerative conditions has increased markedly. Clearer connections have been made between some of the key factors present in patients who have experienced a traumatic brain injury or live with diseases such as dementia, Alzheimer’s or Parkinson’s.
In recent years, inflammation has increasingly been appreciated to be part of the cause of neurodegeneration as opposed to merely being a result of it. These important discoveries published in scientific literature have shifted the way we view brain-related diseases and, thus, the way they can be treated.
Systemic Inflammation and Cognitive Decline
Systemic inflammation is a common factor in diseases of aging—this covers a broad spectrum of severe, debilitating and, sometimes, life-threatening conditions.[1] Scientists researching brain diseases now widely recognize that widespread inflammation is involved in the detrimental process of Parkinson’s disease[2] as well as dementia and, specifically, its most prevalent form which is Alzheimer’s disease (AD).[3]
An informative study conducted in 2009 established a strong association between levels of inflammation and symptoms. 300 mild-to-severe Alzheimer’s patients were assessed with the help of their caregivers for a baseline level of cognitive function, and tested for levels of a molecule (cytokine) which causes inflammation (pro-inflammatory) called Tumor Necrosis Factor- alpha (TNF-a).
Researchers then recorded any new acute systemic inflammatory event, such as an illness or physical trauma that occurred subsequent to the initial testing and retested cognitive function and the TNF-A levels at 2, 4 and 6 months to compare to the baseline levels noted at the beginning of the study. The bottom line was that both acute and chronic systemic inflammation, as measured by increases in the pro-inflammatory TNF-a levels, correlated quite strongly with an increase in cognitive decline in the patients in the study.[4]
Inflammatory-Immune Response
Neurological dysfunction begins with a trigger such as a trauma (as in boxing or other traumatic brain injury), stroke, infection, lifestyle choices (e.g. smoking), environmental toxins, hereditary factors or a combination thereof. One or more of these factors leads to neuroinflammation[5], defined as chronic and persistent inflammation in the central nervous system (CNS) and the brain.
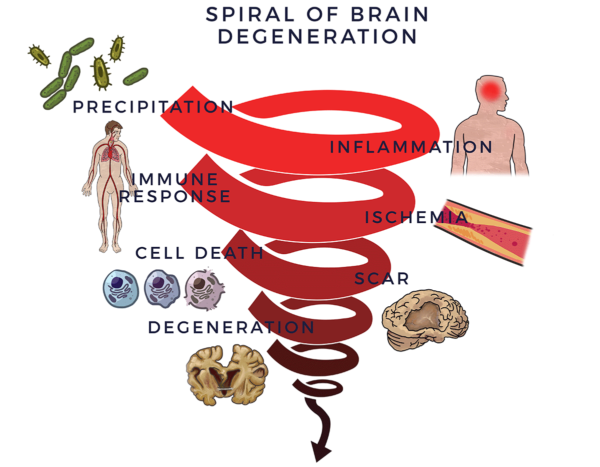
In order to protect the brain, the immune system sets a response in motion. When this process goes out of control, the immune response becomes tantamount to having a backseat driver who is chronically overreacting while “helping” you drive your car. This is also called an inflammatory-immune response and leads to reduced blood flow (ischemia) in the brain. Without good circulation bringing oxygen and essential nutrients to keep the brain healthy[6], nerve cells die off. Scars, plaques or proteins replace healthy tissue and the brain degenerates. We call this process the Spiral of Degeneration.
Loss of neurons (nerve cells) affects balance, movements, talking, breathing and memory in various ways and at different time points depending on the specific diagnosis and rate of deterioration. Parkinson’s Disease is attributed to the loss of nerve cells that produce a chemical called dopamine in the brain. Lack of dopamine causes tremors, balance, movement and speech disorders. It can also lead to depression and poor memory.
The factors of the Spiral are increasingly recognized to lead to the symptoms and decline in the activities of daily living that are the result of brain-related diseases. [7] [8] [9]
The brain is an incredibly resilient organ. By the time a condition such as Parkinson’s or dementia (and associated amyloid plaques, tau proteins or Lewy bodies) becomes apparent, these things may have been in development over a decade or more in a progressively worsening sequence events.
Process of Repair
Through a mechanism of cell-to cell communication known as paracrine signaling[10], adipose-derived stem and regenerative cells (ADRCs) work to mobilize nearby cells to work more efficiently.
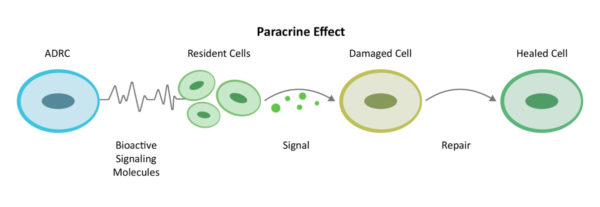
ADRCs also activate the resident cells already at the site inflammation and injury in the brain—but asleep on the job—to get back to work doing their part. These resident cells first decrease inflammation and the overactive immune responses. Once the backseat driving from the immune system lets up they increase circulation with new blood vessel growth and restore health to existing blood vessels, prevent further programmed cell death (apoptosis), decrease scar size and finally regenerate healthy tissue and nerves.
We call this the Process of Repair. The body naturally heals from injury this way, and the healing processes are supported further by the multiple mechanisms of action of ADRCs towards the goal of repair and regeneration in the brain as well as systemic inflammation under control.
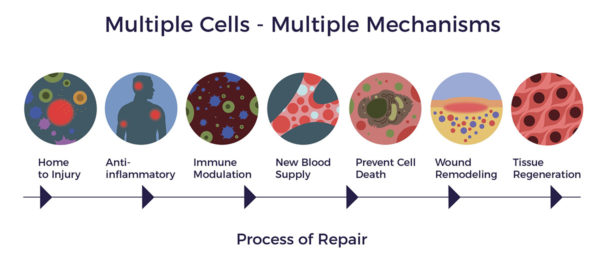
As a result of the multiple activities the ADRCs perform, new blood vessels can form and new neurons in the brain can be generated.[11] [12] As demonstrated in a recent animal study, ADRCs have also been seen to reduce lesions in the brain (as in cases of stroke or brain injury) and thus positively impact learning and memory.[13]
Multiple Cells, Multiple Functions
Unlike a pharmaceutical drug that relies on a single molecule or chemical to subdue the most prominent factor in a disease—such as the multitude of drugs that have been developed to address the amyloid build up, all of which have failed in clinical trials—ADRCs blanket a diseased tissue or organ with hundreds of biologically active molecules that promote cellular, nerve and tissue repair where needed.[14] [15] In other words, we are not relying on a star player with a specific specialty but rather on an entire team of equally competent players.
Mesenchymal stem cells (MSCs), a type of stem cell found in fat, secrete helpful chemicals and growth factors that are known to promote neural cell survival and regeneration through paracrine signaling. This is an important function which promotes various aspects of recovery in the injured brain through decreasing cell death, increasing the growth of new nerve tissue as well as the formation of new blood vessels.[16]
An important group of bioactive molecules released from ADRCs are called neurotrophic factors (NTFs); “neuro” relating to nerve, and “trophic” from Ancient Greek trophikós meaning “pertaining to food or nourishment.” NTFs support the growth, survival, and differentiation of both developing and mature nerve cells (neurons). It has been shown that adipose-derived stem cells (ADSCs) release brain-derived neurotrophic growth factor (BDNF) which promotes nerve healing and axon growth.[17] [18] [19] [20]
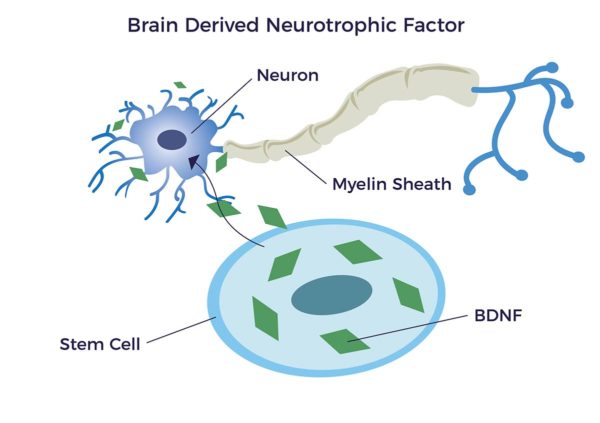 Higher levels of BDNF are associated with increased intelligence, mood, productivity, and memory along with decreased risks of dementia and Alzheimer’s disease.
Higher levels of BDNF are associated with increased intelligence, mood, productivity, and memory along with decreased risks of dementia and Alzheimer’s disease.
Permeating the Blood-Brain Barrier Blood-Brain-Barrier
Standing between the brain and exterior foreign substances is the blood brain barrier (BBB) whose job it is to act as a filter and to maintain a stable environment for the brain. In the treatment of neurodegenerative disorders, AMBROSE doctors deliver a low dose of mannitol, a sugar alcohol, intravenously prior to stem cell infusion. Mannitol temporarily opens up the blood brain barrier so that a significantly higher percentage of cells can safely migrate into the brain[21] and is what doctors first use to relieve the brain of excess fluid or infections as well as prior to IV infusion of cancer drugs.
AMBROSE Cell Therapy for Brain Injury and Neurodegenerative Disorders
Adipose-Derived Stem and Regenerative Cells (ADRCs) are a diverse population of cells that, through multiple activities, have the potential to help improve the symptoms, function and quality of life for patients with brain-related disorders or who have experienced a traumatic brain injury.[22] [23]
These cells are akin to having a personalized fire, rescue and repair crew that naturally resides in your body. They wait quietly for a sign of trouble—inflammation—and then home to that signal to do their jobs.
By harnessing the power of your own biology, AMBROSE Cell Therapy represents a minimally invasive option for patients with neurodegenerative conditions such as Parkinson’s, dementia and Alzheimer’s Disease, or after a stroke or traumatic brain injury.
Please contact us for more information about treatment, candidacy and how to become a patient.
[1] C. Franceschi and J. Campisi Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age- Associated Diseases J Gerontol A Biol Sci Med Sci 2014 June;69(S1): S4–S9
[2] C. Ferrari and R. Tarelli. Parkinson’s Disease and Systemic Inflammation Parkinson’s Dis. 2011; 2011: 436813.
[3] R Schmidt & H Schmidt et al Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia Aging Study. 2002 Annals of neurology. 52. 168-74. 10.1002/ana.10265
[4] C. Holmes et al Systemic inflammation and disease progression in Alzheimer disease Neurology® 2009; 73:768–774
[6] E Hirsch and S Hunot Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 2009; 8: 382–97
[6] RN Kalaria (2010). Vascular Basis for Brain Degeneration: Faltering Controls and Risk Factors for Dementia. Nutrition Reviews, 68(Suppl 2), S74–S87
[7] BV Zlokovic Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature reviews Neuroscience. 2011;12(12):723-738
[8] Linda J. Van Eldik et al The roles of inflammation and immune mechanisms in Alzheimer’s disease Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2 (2016) 99-109
[9] Kim S, Chang K-A, Kim Ja, Park H-G, Ra JC, et al. (2012) The Preventive and Therapeutic Effects of Intravenous Human Adipose-Derived Stem Cells in Alzheimer’s Disease Mice. PLoS ONE 7(9): e45757.
[10] A Caplan MSCs: The Sentinel and Safe-Guards of Injury J. Cell. Physiol. 231: 1413–1416, 2016
[11] J. K. Huang et al Myelin Regeneration in Multiple Sclerosis: Targeting. Endogenous Stem Cells., The American Society for Experimental NeuroTherapeutics, Inc. 2011
[12] A Bowles et al Immunomodulatory Effects of Adipose Stromal Vascular Fraction Cells Promote Alternative Activation Macrophages to Repair Tissue Damage. Stem Cells, 2017 Aug 12
[13] Zhou F, Gao S, Wang L, et al. Human adipose-derived stem cells partially rescue the stroke syndromes by promoting spatial learning and memory in mouse middle cerebral artery occlusion model [published correction appears in Stem Cell Res Ther. 2019 Mar 6;10(1):76]. Stem Cell Res Ther. 2015;6(1):92. Published 2015 May 9. doi:10.1186/s13287-015-0078-1
[14] Takeshi Taksuda et al Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes Scientific Reports 3: 1197
[15] A Brini et al, Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain Scientific Reports 7: 9904
[16] C. Tate and C Case. Mesenchymal Stromal Cells to Treat Brain Injury. Advanced Topics in Neurological Disorders.
[17] Razavi, Shahnaz et al. “Neurotrophic Factors and Their Effects in the Treatment of Multiple Sclerosis.” Advanced Biomedical Research 4 (2015): 53. PMC. Web. 28 Sept. 2018.
[18] J. K. Huang et al Myelin Regeneration in Multiple Sclerosis: Targeting. Endogenous Stem Cells., The American Society for Experimental NeuroTherapeutics, Inc. 2011
[19] T Lopatina et al. (2011) Adipose-Derived Stem Cells Stimulate Regeneration of Peripheral Nerves: BDNF Secreted by These Cells Promotes Nerve Healing and Axon Growth De Novo. PLoS ONE 6(3): e178991
[20] S. Seigo et al, Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration, Journal of Orthopaedic Science, Volume 18, Issue 1,2013, Pages 145-151
[21] C.V. Borlongan et al Permeating the Blood Brain Barrier and Abrogating the Inflammation in Stroke: Implications for Stroke Therapy Curr Pharm Des. 2012; 18(25): 3670–3676
[22] Sakthiswary R, Raymond AA. Stem cell therapy in neurodegenerative diseases: From principles to practice. Neural Regen Res. 2012;7(23):1822–1831. doi:10.3969/j.issn.1673-5374.2012.23.009
[23] Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70(3):353–361. doi:10.1002/ana.22487