
A Golden Era of Cell-Assisted Resilience
A Golden Era of Cell-Assisted Resilience
RJ’s Patient-Reported Outcome
Sports-related injuries, including concussions and whiplash, three failed shoulder surgeries, an unsuccessful right knee surgery, etc., retired RJ’s dream of playing Major League Baseball.
In May 2022, he exercised his Right to Try AMBROSE Cell Therapy. Seven months later, he says, “Y’all (AMBROSE) changed my life.”
RJ’s Story RJ played baseball, football, snowboarding, and golf growing up.
RJ played baseball, football, snowboarding, and golf growing up.
“I had some concussions and whiplash from football and a few car accidents along the way.”
RJ’s shoulder issues started in high school. Initially, his doctor thought it was bicep tendonitis. “I had to take a month off every year. It never felt good.”
One issue led to another. “When I was 17, I blew out my left knee running second base. After that, it would give out a lot.” The MRI showed his femur had lengthened, but it had never bothered RJ before the spill.
RJ’s orthopedic surgeon shaved the femur down and cleaned up the knee. “After the surgery, I did P.T. My knee felt better. I could run but couldn’t walk downhill or stairs.”
Jumping ahead, before his AMBROSE Cell Therapy, RJ complained of left knee pain, clicking, and inability to walk downstairs or a hill. His right knee was symptomatic from compensating. “I could run, but it hurt,” he later said.
Resuming RJ’s story: “I went back to playing baseball. My shoulder gave out after the first couple of days.”
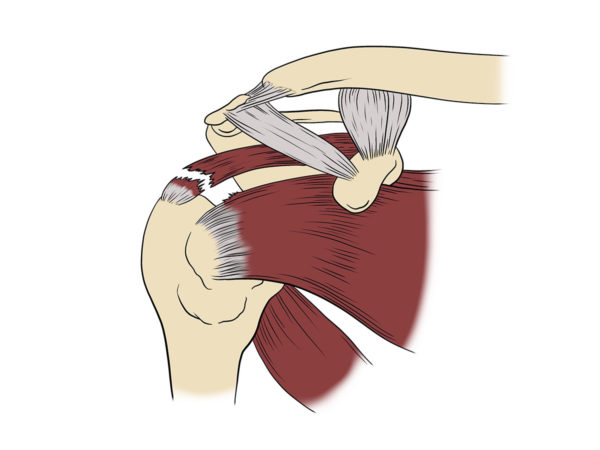 “Nothing showed up on my MRI and X-Ray, but I couldn’t move my arm. I went in for exploratory surgery. The surgeon found my rotator cuff and labrum were shredded.
“Nothing showed up on my MRI and X-Ray, but I couldn’t move my arm. I went in for exploratory surgery. The surgeon found my rotator cuff and labrum were shredded.
He repaired it, but then every season, it would give out”, RJ recounted.
Doctors refer to the shoulder joint’s major tendons as the rotator cuff. Rotator cuff tears are common injuries. Advances in surgery have improved rotator cuff repairs. But failure rates remain high.
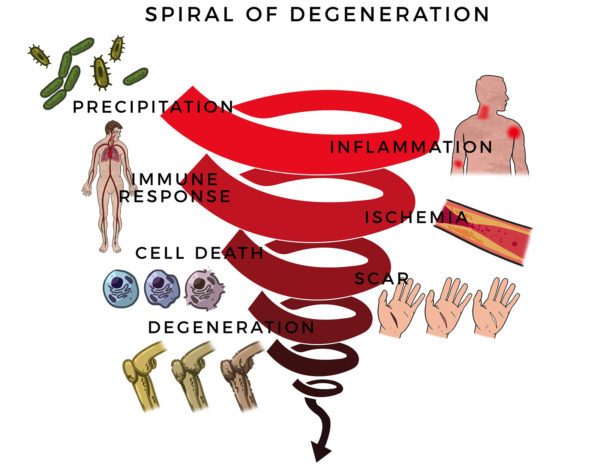 Here, RJ’s massive rotator cuff tear (MRCT) caused disability and pain. An MRCT precipitates a Spiral of Degeneration beginning with inflammation, abnormal immune response, and lack of blood flow. That leads to programmed cell death (apoptosis), scarring, and degeneration.
Here, RJ’s massive rotator cuff tear (MRCT) caused disability and pain. An MRCT precipitates a Spiral of Degeneration beginning with inflammation, abnormal immune response, and lack of blood flow. That leads to programmed cell death (apoptosis), scarring, and degeneration.
Unknown to many people and a key reason shoulder surgeries have such a high failure rate, the muscle around the rotator cuff shrinks, and the body replaces it with fatty tissue.
When the tendon and muscle are finally reattached surgically to the shoulder bone, the weakened muscle can’t handle everyday stresses, and the area can be re-injured.
“I tore my rotator cuff and labrum twice more, each followed by another surgery. Both surgeries failed. In between, I ripped a hamstring, broke some fingers, and fractured my hand while stealing second base,” he said.
Cortisone injections, PRP, and physical therapy failed to bring lasting relief.
In his senior year in college and after the last surgery, his baseball coach discouraged RJ from returning to the team.
Allostatic Load and Anti-resilience.
Sterling and Eyer introduced the concept of allostasis in 1988 as stability through change. From the Greek állos, “other,” “different” + stasis, “standing still.” The researchers were imparting the concept of “remaining stable by being variable.”.
Just as a sailor keeps his boat on an even keel despite choppy waters, the body’s biological systems maintain their equilibrium (homeostasis), despite being put through stress.
In contrast, allostatic Load is “the wear and tear on the body” accumulated from repeated or chronic stress. (Bruce McEwen and Eliot Stellar 1993) Sailing across tall breaking waves over and over can cause irreparable damage.
After RJ’s sports injuries, concussions, surgeries, and competitive stress, his physiologic systems were striking out. He descended from an elite baseball player to being told by his college coach that the disabled list was not a viable option.
By age 23, RJ experienced debilitating symptoms beyond losing his ability to throw with his right arm and run the bases. He stopped bouncing back. In other words, allostatic load set in.
Baseline
RJ’s constellation of wear and tear complaints included:
- Right shoulder pain, decreased mobility, and left shoulder stiffness.
- Right elbow stiff, made clicking noises, left elbow stiffness though no pain, no clicking
- Bilateral arms, decreased reflexes with hammer exam
- Right knee, sharp pain with walking, early knee fatigue with running, aches in cold weather.
- Left knee clicking, popping, and stiffness from compensating
- Challenging to walk downstairs or declines.
- Lower neck – intermittent numbness and radiation of numbness and tingling into the arm
- Right upper arm, numbness near upper biceps insertion, constant
- Right upper arm to fingers (middle & ring fingers), tingling, intermittent nerve pain
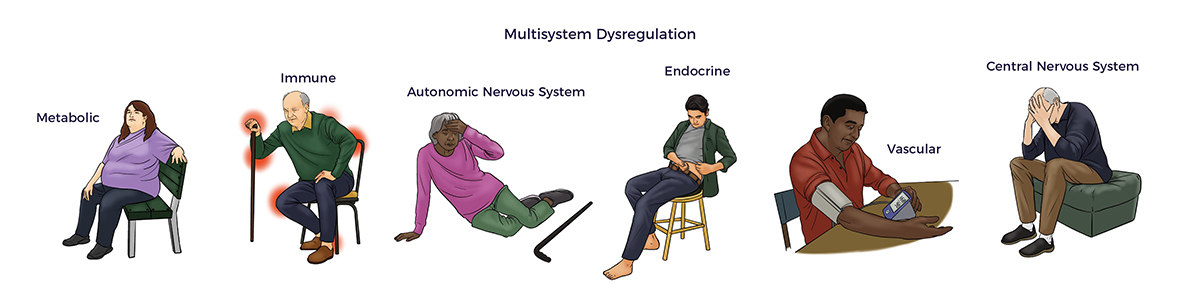
 More concerning, RJ experienced symptoms of multisystem dysregulation:
More concerning, RJ experienced symptoms of multisystem dysregulation:
- Vertigo, when looking rapidly with turning his head
- Daily headaches
- Sleeping 15 hours per day,
- Waking up drenched from night sweats
- Depression
- An extreme tendency to fall asleep (narcolepsy)
- Chronic fatigue
In short, allostatic load was ahead 3-2 with the bases loaded in the bottom of the 9th inning. The odds were stacked against RJ Surgeries and drugs had failed him. Though recommended, RJ had the good sense of avoiding pain meds and psychotropic drugs such as Prozac, which could have worsened matters.
Fortunately, RJ had the Right to Try AMBROSE Cell Therapy.
ADRC-assisted Resilience
Based on abundant literature, AMBROSE hypothesized that ADRC-based therapy could unburden allostatic load.[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11]
Further, preeminent researchers from the Texas Heart Institute, University of Tokyo, Cedar-Sinai, and other respected institutions have published studies that support ADRCs’ potential to treat multiple chronic conditions. [12] [13] [14] [15] [16]
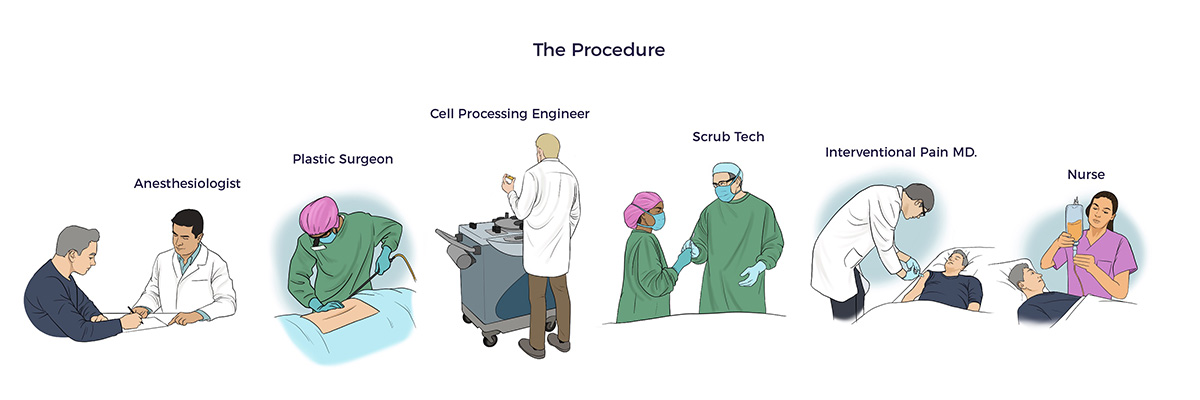
The medical team personalized the AMBROSE Master Protocol to address RJ’s unique health challenges.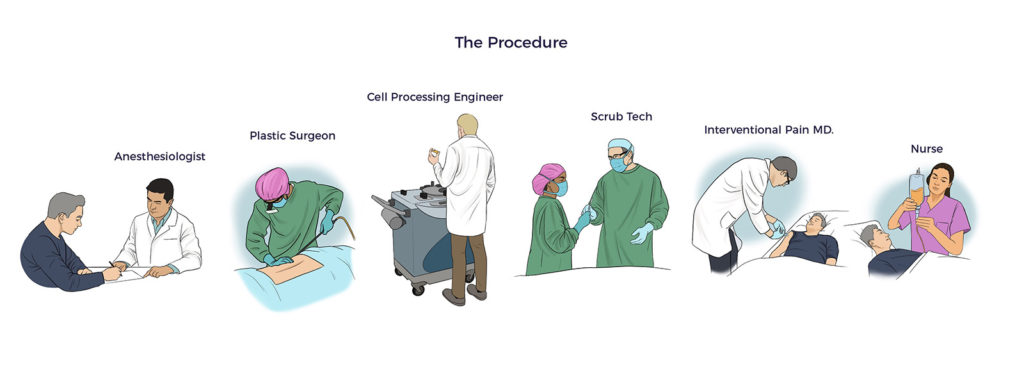
- Fat Harvesting
Using minimally traumatic water-assisted liposuction technology (WAL), AMBROSE’s board-certified plastic surgeon harvested 580 ccs (19 oz) of adipose tissue. Post-procedure, RJ reported using only Tylenol for a few days.
- Spine and Joint Injections
AMBROSE relied on published research in addressing the patient’s cervical spine and diseased joints with PRP-enriched micronized fat injections. [17] [18] [19] [20] [21] [22] [23]
AMBROSE’s fellowship-trained interventional pain specialist delivered 61 precise injections under real-time ultrasound guidance into RJs:
- Inflamed cervical spine (22 injections)
- Arthritic shoulders and bicep tendons (8 injections),
- Sore elbows (3 injections)
- Painful, clicking knees (18 injections).
Results
Seven months out, RJ is playing golf, swinging the baseball bat again, running, walking down declines, lifting weights, and coaching high school football and baseball.
As a result of compensating for his bum shoulder, his right elbow became his biggest problem. “My arm feels really good now- no pain, clicking, or stiffness.” He is throwing the baseball again, though, taking his time to rebuild his deconditioned shoulder muscle.
After his knee surgery, his knees deteriorated. He says, “Running is no problem now; The sharp pain and stiffness are gone. I can walk downstairs and hills again. My friends don’t laugh at me anymore.”
“My neck is much better. I don’t have the numbness and tingling. I don’t get dizzy when I turn it to the side. I haven’t recovered full range of motion yet – but that is getting better too.”, RJ added
- ADRC-IV Infusion
RJ received 129 million Celution™ system processed ADRCs with 92% cell viability through IV infusion.
Published research has established that the ADRC-IV infusion reduces neuroinflammation, improves blood flow, restores autonomic nervous system function, and so on. RJ’s real-world results validate the cited studies. [24] [25] [26]
As an aside, our group’s research revealed the Celution system significantly outperforms the Medikhan, Tissue Genesis Icellator, and other commercially available adipose cell processing systems.[27] [28]
IV Results
In line with the cited publications (and others), RJ started feeling better not long after his AMBROSE treatment.
 “I don’t feel depressed like I did before. I am optimistic and have more energy. I don’t wake up drenched with sweat and only need about eight hours of sleep now. I used to sleep from 12:00 am to 2:00 pm and take an hour nap too. Now I coach baseball and football 15 hours a day.”, RJ reported.
“I don’t feel depressed like I did before. I am optimistic and have more energy. I don’t wake up drenched with sweat and only need about eight hours of sleep now. I used to sleep from 12:00 am to 2:00 pm and take an hour nap too. Now I coach baseball and football 15 hours a day.”, RJ reported.
He went back to the gym, resumed light weightlifting, and lost 15 lbs. – with no change in diet. RJ added, “I haven’t been in this good of shape for at least two years,”
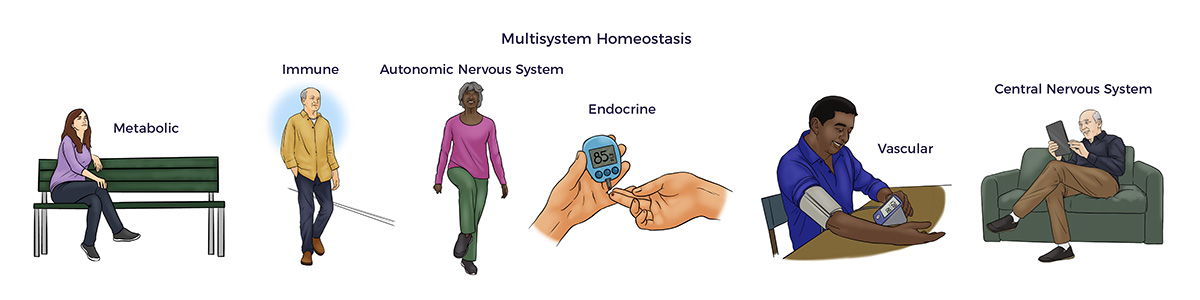
RJ’s benefits signal a reduction in allostatic load and restoration of multisystem homeostasis.
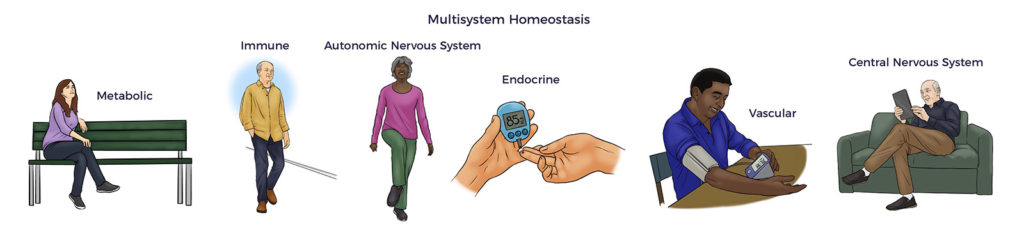
RJ is not alone.
Research studies have established a direct link between increasing allostatic Load and all-cause mortality. Unsurprisingly, studies have also connected heart disease, diabetes, kidney disease, lung disease, and so on with allostatic Load. Thus, beyond RJ’s poor health, he was at high risk of getting worse at a young age. [29]
Today’s environmental, psychological, and lifestyle factors are accelerating the time-to-allostatic Load:
- A University College of London study reveals Gen X faces more years of ill health than Baby Boomers.
- According to a CNBC poll, nearly half of older millennials have at least one chronic disease.
- According to an American Heart Association survey, health concerns weigh heavier on Gen Z, RJ’s age group.
Conclusion
“The doctors and surgery center team were the best I have had. My future has gone from bleak to bright. I appreciate Matt Feshbach, AMBROSE CEO, for his follow-up and friendship. For me, AMBROSE was a grand slam.”, RJ concluded.
[1] Ghachem, A., Fried, L.P., Legault, V. et al. Evidence from two cohorts for the frailty syndrome as an emergent state of parallel dysregulation in multiple physiological systems. Biogerontology 22, 63–79 (2021).
[2] Gross, Alden L et al. “Derivation of a measure of physiological multisystem dysregulation: Results from WHAS and health ABC.” Mechanisms of ageing and development vol. 188 (2020): 111258.
[3] Guidi J, Lucente M, Sonino N, Fava G, A: Allostatic Load and Its Impact on Health: A Systematic Review. Psychother Psychosom 2021;90:11-27.
[4] Hirose Y et al. Comparison of trophic factors secreted from human adipose-derived stromal vascular fraction with those from adipose-derived stromal/stem cells in the same individuals Cytotherapy, 2018; 20: 589–591
[5] VL Negenborn et al. Autologous Fat Grafting as a Last Resort for Unsustainable Pain in a Woman with Multiple Osteochondromas Archives of Plastic Surgery Vol. 44 No. 2 March 2017
[6] S Tamburino et al The Role of Nanofat Grafting in Vulvar Lichen Sclerosus: A Preliminary Report Arch Plast Surg 2016;43:93-95
[7] H Riyat et al Autologous fat grafting for scars, healing and pain: a review Scars, Burns & Healing Volume 3: 1–
[8] T Lopatina et al. (2011) Adipose-Derived Stem Cells Stimulate Regeneration of Peripheral Nerves: BDNF Secreted by These Cells Promotes Nerve Healing and Axon Growth De Novo. PLoS ONE 6(3): e178991
[9] S. Seigo et al, Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration, Journal of Orthopaedic Science, Volume 18, Issue 1,2013, Pages 145-151
[10] Blaszkiewicz, M., Wood, E., Koizar, S. et al. The involvement of neuroimmune cells in adipose innervation. Mol Med 26, 126 (2020)
[11] F Caviggioli, M.D. Autologous Fat Graft in Postmastectomy Pain Syndrome Plastic and Reconstructive Surgery August 2011
[12] S. Kesten and JK Fraser Autologous Adipose Derived Regenerative Cells: A Platform for Therapeutic Applications Advanced Wound Healing Surgical Technology International XXIX
[13] Nguyen A et al. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature Journal of Plastic, Reconstructive & Aesthetic Surgery (2016) 69, 170e179
[14] Guo J et al. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action Journal of Plastic, Reconstructive & Aesthetic Surgery (2016) 69, 180e188
[15] Al-Ghadban S, Artiles M, Bunnell BA. Adipose Stem Cells in Regenerative Medicine: Looking Forward. Front Bioeng Biotechnol. 2022; 9:837464. Published 2022 Jan 13.
[16] J. Willerson and E. Perin Buying New Soul J Am Coll Cardiol. 2012;60(21):2250-2251
[17] Ibrahim, Samir, Rybacka-Mossakowska, Joanna and Michalak, Sławomir. “Fat graft – the natural choice for reconstructive, regenerative and aesthetic surgery” Medical Journal of Cell Biology, vol.5, no.2, 2017, pp.113-117. https://doi.org/10.1515/acb-2017-0008
[18] Heather Vinet-Jones*,1 & Kevin F Darr Clinical use of autologous micro-fragmented fat progressively restores pain and function in shoulder osteoarthritis Regen.Med. (2020) 15(10), 2153–2161
[19] Striano Rd, Malanga G, Bilbool N, Azatullah K. Refractory shoulder pain with osteoarthritis, and rotator cuff tear, treated with micro-fragmented adipose tissue. J. Orthopaedics Spine Sports Med. 2(1), 14–19 (2018).
[20] Lädermann A, Denard PJ, Burkhart SS. Management of failed rotator cuff repair: a systematic review. J ISAKOS. 2016;1(1):32-37. doi:10.1136/jisakos-2015-000027
[21] Heather Vinet-Jones & Kevin F Darr Clinical use of autologous micro-fragmented fat progressively restores pain and function in shoulder osteoarthritis Future Medicine Ltd Regenerative Medicine Volume 15, Issue 10, October 2020, Pages 2153-2161
[22] D.M. Robinson, C. Eng, M. Mitchkash, A.S. Tenforde, J. Borg-Stein Outcomes after Micronized Fat Adipose Transfer for Glenohumeral Joint Arthritis and Rotator Cuff Pathology: a Case Series of 18 Shoulders Muscles, Ligaments and Tendons Journal 2020;10 (3)
[23] Itro, A. et al. Why Use Adipose-Derived Mesenchymal Stem Cells in Tendinopathic Patients: A Systematic Review. Pharmaceutics 2022, 14, 1151.
[24] J Rosenstein, J Krum & C Ruhrberg VEGF in the nervous system Organogenesis 6:2, 107-114; April/May/June 2010; © 2010 Landes Bioscience
[25] Numan MT et al. Autologous Adipose Stem Cell Therapy for Autonomic Nervous System Dysfunction in Two Young Patients. Stem Cells and Development 2017 26:6, 391-393
[26] J. Vaquero et al Progressive increase in brain glucose metabolism after intrathecal administration of autologous mesenchymal stromal cells in patients with diffuse axonal injury Cytotherapy, 2018; 20: 806–819
[27] A Caplan PhD Mesenchymal Stem Cells J Orthop Res, Vol. 9, No. 5, 1991
[28] Skok M. Mesenchymal stem cells as a potential therapeutic tool to cure cognitive impairment caused by neuroinflammation. World J Stem Cells 2021; 13(8): 1072-1083
[29] P.G. Shiels et al. Circulating markers of ageing and allostatic load: A slow train coming Practical Laboratory Medicine 7 (2017) 49–5451







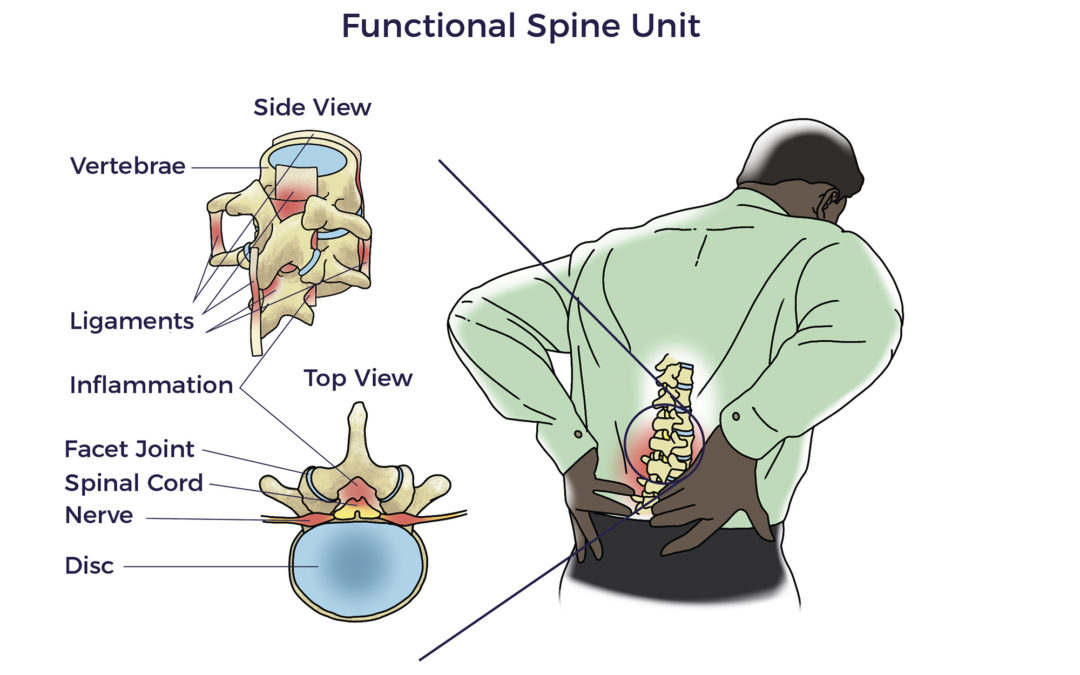
 Spine care has its origins in antiquity. The Edwin Smith surgical papyrus, an Egyptian document written in the 17th century BC, is the first known discussion of neck and back-related injuries. Hippocrates (4th century BC) experimented with traction or local pressure to correct spinal deformities. Aristotle also contributed to our current day understanding of the neck and spine.
Spine care has its origins in antiquity. The Edwin Smith surgical papyrus, an Egyptian document written in the 17th century BC, is the first known discussion of neck and back-related injuries. Hippocrates (4th century BC) experimented with traction or local pressure to correct spinal deformities. Aristotle also contributed to our current day understanding of the neck and spine.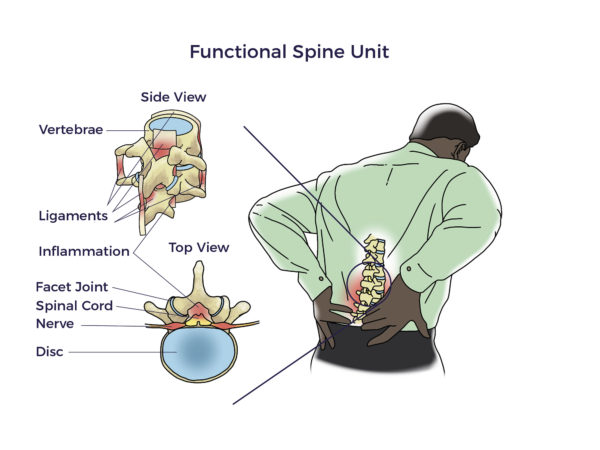 But there was still more to be discovered than the FSU. More recently, researchers began focusing on the vicious interplay involved with traumatic injuries, wear and tear, inflammation, and other diseases (co-morbidities). For example, patients with heart disease, diabetes, neurologic conditions, and autoimmune diseases have a higher prevalence of spine disorders than others without those conditions. In fact, Barb, Trish, Kathy, and Jeff each lived with other chronic debilitating conditions, including arthritis, hypermobility, kidney failure, and spinal cord injury, respectively.
But there was still more to be discovered than the FSU. More recently, researchers began focusing on the vicious interplay involved with traumatic injuries, wear and tear, inflammation, and other diseases (co-morbidities). For example, patients with heart disease, diabetes, neurologic conditions, and autoimmune diseases have a higher prevalence of spine disorders than others without those conditions. In fact, Barb, Trish, Kathy, and Jeff each lived with other chronic debilitating conditions, including arthritis, hypermobility, kidney failure, and spinal cord injury, respectively. 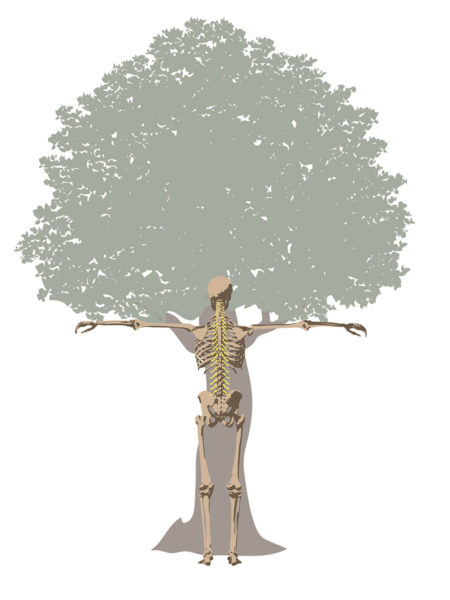 Circling back, in the early 1500s, Leonardo da Vinci took an unusual interest in tree anatomy. His Rule of Trees explained the balance between the trunk and branches of a tree. He counted the rings in tree trunks to determine “the nature of past seasons.”
Circling back, in the early 1500s, Leonardo da Vinci took an unusual interest in tree anatomy. His Rule of Trees explained the balance between the trunk and branches of a tree. He counted the rings in tree trunks to determine “the nature of past seasons.”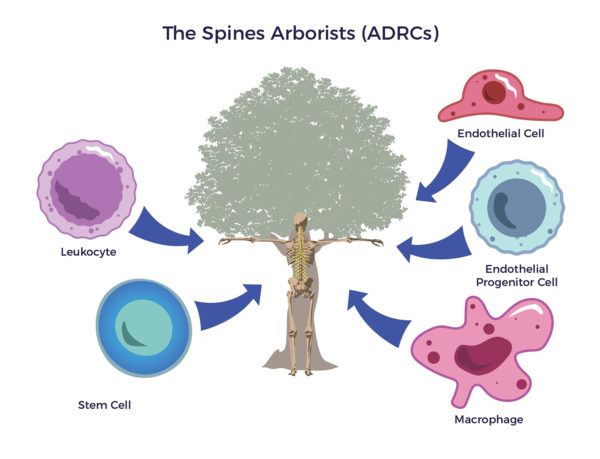 An arborist cultivates trees; tree removal is a last resort. The first thing that goes through their minds is how to save the tree. To do their jobs, arborists cultivate the whole tree. They use fertilizer, irrigation, and other regenerative tools to restore the tree’s trunk, limbs, and leaves.
An arborist cultivates trees; tree removal is a last resort. The first thing that goes through their minds is how to save the tree. To do their jobs, arborists cultivate the whole tree. They use fertilizer, irrigation, and other regenerative tools to restore the tree’s trunk, limbs, and leaves.
 Muhammed Ali’s 1960 Olympic Gold medal started his ascent to being “The Greatest,” but the countless blows to his head – which he welcomed to tire his opponent out – ended in a years-long failed fight to save his brain.
Muhammed Ali’s 1960 Olympic Gold medal started his ascent to being “The Greatest,” but the countless blows to his head – which he welcomed to tire his opponent out – ended in a years-long failed fight to save his brain.
 In 2000, Tim retired from offshore powerboat racing with four world speed records. He once hit upwards of 180 mph.
In 2000, Tim retired from offshore powerboat racing with four world speed records. He once hit upwards of 180 mph. Essential tremor is a nervous system (neurological) disorder that causes involuntary and rhythmic shaking. It can affect almost any part of your body, but the trembling occurs most often in your hands — especially when you do simple tasks, such as drinking from a glass, handwriting, or tying shoelaces.
Essential tremor is a nervous system (neurological) disorder that causes involuntary and rhythmic shaking. It can affect almost any part of your body, but the trembling occurs most often in your hands — especially when you do simple tasks, such as drinking from a glass, handwriting, or tying shoelaces. “I must have had 1,000 concussions while racing. I probably went unconscious 100 times behind the wheel from the G-forces,” Tim estimated.
“I must have had 1,000 concussions while racing. I probably went unconscious 100 times behind the wheel from the G-forces,” Tim estimated.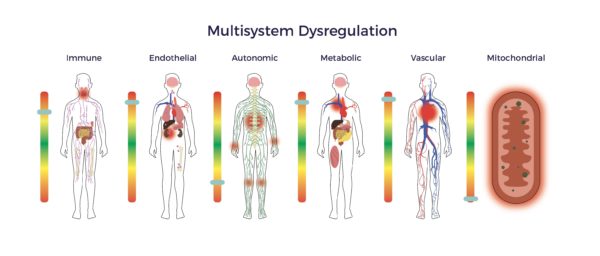
 To solve for this, doctors prescribe patients drugs for each ailment or symptom, e.g., Sinemet for Parkinson’s, Ambien for sleep, steroids for arthritis, opioids for pain, and so forth.
To solve for this, doctors prescribe patients drugs for each ailment or symptom, e.g., Sinemet for Parkinson’s, Ambien for sleep, steroids for arthritis, opioids for pain, and so forth.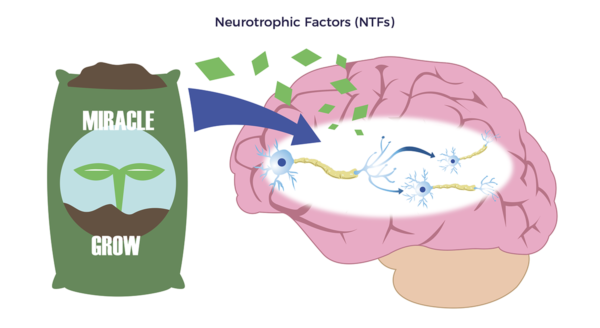
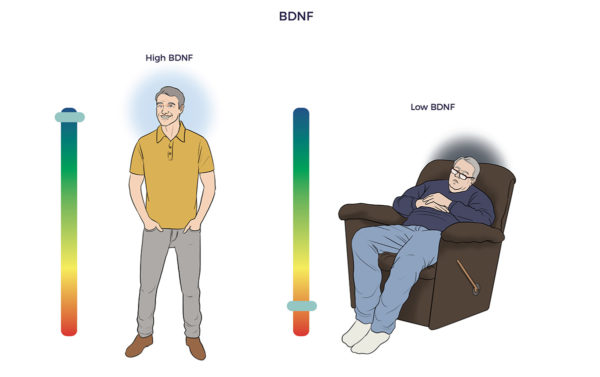 John Hopkins researchers developed a BDNF-blood test that could have predicted the severity of Ali’s head damage and how he would fare. Their study showed that patients with brain injuries have less than one-third of the BDNF as those with healthy brains.
John Hopkins researchers developed a BDNF-blood test that could have predicted the severity of Ali’s head damage and how he would fare. Their study showed that patients with brain injuries have less than one-third of the BDNF as those with healthy brains.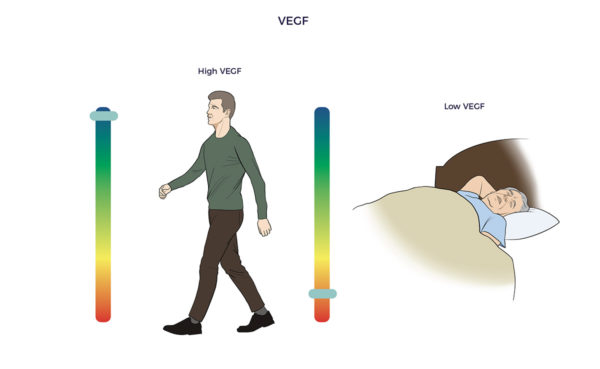 ADRCs also enrich the brain with vascular endothelial growth factor (VEGF). VEGF restores blood flow and reduces inflammation in withering tissues, blood vessels, and organs. One study found higher levels of VEGF in asymptomatic seniors who died with amyloid plaques compared to symptomatic Alzheimer’s patients.
ADRCs also enrich the brain with vascular endothelial growth factor (VEGF). VEGF restores blood flow and reduces inflammation in withering tissues, blood vessels, and organs. One study found higher levels of VEGF in asymptomatic seniors who died with amyloid plaques compared to symptomatic Alzheimer’s patients.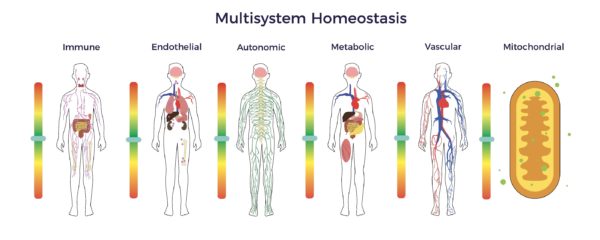
 Since December 2019, SARS coronavirus 2 (SARS-CoV-2) has spread like wildfire across the globe. Unlike the seasonal flu or cold, COVID-19 can attack all body systems and multiple organs.
Since December 2019, SARS coronavirus 2 (SARS-CoV-2) has spread like wildfire across the globe. Unlike the seasonal flu or cold, COVID-19 can attack all body systems and multiple organs.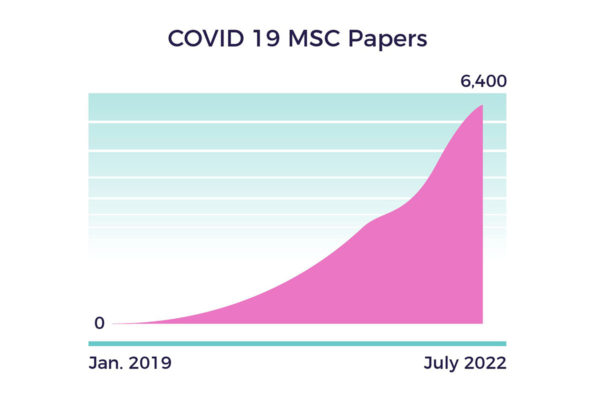
 The in-human MSC studies included Covid-induced:
The in-human MSC studies included Covid-induced: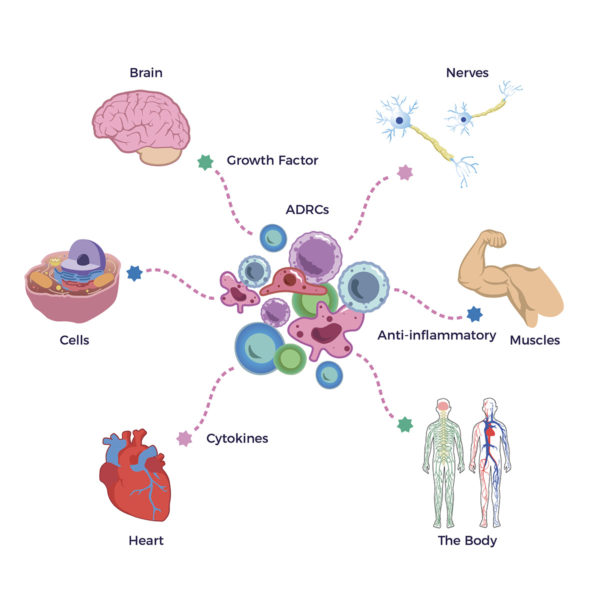 Most promising for Brian, his long-Covid symptoms intersected with those from hypermobility, concussions, renal failure, Lyme Disease, and post-chemo side effects.
Most promising for Brian, his long-Covid symptoms intersected with those from hypermobility, concussions, renal failure, Lyme Disease, and post-chemo side effects. Brian experienced classic long-hauler symptoms: brain fog, chronic fatigue, and emotional volatility. As he summarized, “Most of 2021 was a lost year.”
Brian experienced classic long-hauler symptoms: brain fog, chronic fatigue, and emotional volatility. As he summarized, “Most of 2021 was a lost year.”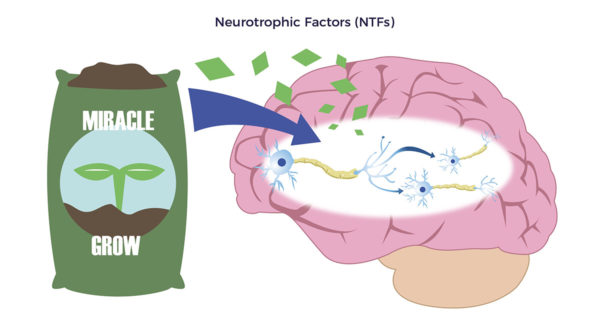 Supporting Brian’s statements, ADRCs release “Neurotrophic factors (NTFs),” Neuro relating to nerve and trophic, from Ancient Greek trophikós, meaning” of food or nourishment.” NTFs, through multiple mechanisms, reduce neuroinflammation and stimulate the development of new brain cells, brain cell connections, and nerves.
Supporting Brian’s statements, ADRCs release “Neurotrophic factors (NTFs),” Neuro relating to nerve and trophic, from Ancient Greek trophikós, meaning” of food or nourishment.” NTFs, through multiple mechanisms, reduce neuroinflammation and stimulate the development of new brain cells, brain cell connections, and nerves. 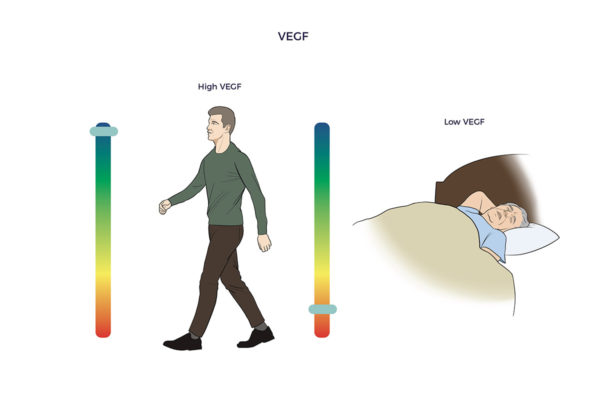 Covid left Brian with debilitating inflamed blood vessels and swelling in both legs, or vasculitis, an autoimmune condition. He also complained of constant numbness and tingling in both legs (neuropathy). The right leg was worse than the left.
Covid left Brian with debilitating inflamed blood vessels and swelling in both legs, or vasculitis, an autoimmune condition. He also complained of constant numbness and tingling in both legs (neuropathy). The right leg was worse than the left.


 Recommending AMBROSE to Family and Friends
Recommending AMBROSE to Family and Friends