Cell Therapy Procedure Protocol
AMBROSE takes a personalized approach to your cycle of care – beginning with your initial consultation with our MD patient consultant, through the cell therapy procedure itself, and suggested after-care plans.
Individualized treatment plans are uniquely developed with each patient’s goals in mind. AMBROSE procedure protocols each consist of:
- Tissue harvesting,
- Direct injections (when called for), and
- ADRC preparation and cell delivery.
Intravenous (IV) infusion of ADRCs is the approach used to target the chronic systemic inflammation (inflammaging), which underlies the conditions AMBROSE protocols address. Additionally, biodistribution studies have documented that adult stem cells delivered IV migrate through the lungs to all of the organs of the body. AMBROSE’s comprehensive treatment plans, most often but not always, include direct injections into inflamed tissues affected by or accompanying the underlying the inflammaging.
By way of example, certain conditions including but not limited to those related to the lungs, kidney, liver, or brain, are best addressed with IV administration only. In comparison, a number of autoimmune diseases, such as RA, lupus, and psoriatic arthritis, as well as neuropathies, will include localized injections.
Our physicians are board-certified in the specialties required to harvest the fat safely and deliver ADRCs effectively to the needed targets.
This is a same-day, out-patient procedure that takes approximately 4 hours including 2.5 hours to process the ADRCs in the Celution cell processing system.
Phase 1: Tissue Harvesting
All AMBROSE care plans begin with a fat harvesting procedure, performed by a board-certified plastic surgeon, utilizing body-jet® EVO water-assisted liposuction (WAL) technology. Using this innovative system ensures minimal trauma for the patient and is also documented to be a tissue and cell-friendly fat harvesting method. Water-assisted liposuction differs greatly from conventional liposuction methods. A tissue harvest done using the body-jet® system gently detaches fat from the tissue structure with the help of a pulsating water jet. The result is a minimized risk of bruising and swelling, shorter recovery time and heightened patient satisfaction with the secondary cosmetic outcome. 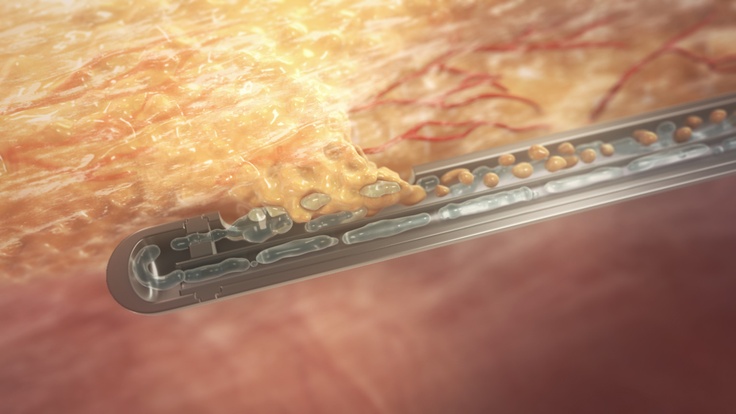 Most importantly, surrounding tissue, nerves and vascular structures sustain much less damage with the EVO system. Sparing tissue and avoiding extensive trauma is highly relevant to achieving and even enhancing the therapeutic outcome of cell therapy.
Most importantly, surrounding tissue, nerves and vascular structures sustain much less damage with the EVO system. Sparing tissue and avoiding extensive trauma is highly relevant to achieving and even enhancing the therapeutic outcome of cell therapy.
Unique to the EVO system, further cleaning and preparation of the harvested tissue (lipoaspirate) for cell processing or direct injection, is not necessary. The lipoaspirate is already optimized for both liberating the ADRCs from it (using the Celution System) for the IV delivery as well as being prepared for small volume injections of the tissue (with the ADRCs remaining resident within it) for musculoskeletal injection.
Phase 2: Cell Processing and Separation
All AMBROSE care plans include the delivery of adipose-derived stem and regenerative cells (ADRCs) via IV infusion, a step which calls for the use of the closed, sterile Celution® System for cell processing. Integrated into the Celution® process and protocol is the use of a non-mammalian based enzyme which digests the adipose tissue and liberates the ADRCs. A second enzyme is also used that separates the cells from one another and prevents clumping. Finally, the resultant cell product is triple-washed to remove any residual enzyme or other debris. With the above completed, the ADRC preparation is ready for IV infusion or direct injection if called for in the treatment plan. 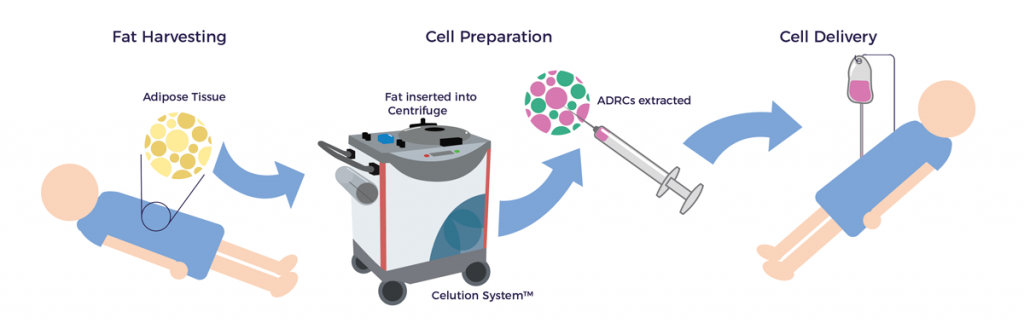
Phase 3a: Ultrasound or Fluoroscopy-Guided Direct Injections
For those patients whose treatment plans call for direct injections, the adipose tissue is enriched with platelet-rich plasma (PRP) prior to injection. PRP releases growth factors – various proteins that promote the growth, organization, and maintenance of cells and tissues. [1] PRP has a stimulating effect on the stem cells in fat and improves their retention, so they work harder and longer. [2]
Using ultrasound or fluoroscopy guidance at the point of care, direct injection sites are mapped out. The PRP-enriched fat is then delivered to the target sites according to the individualized care plan. AMBROSE injection protocols target the inflamed joints, muscles, ligaments, tendons and nerve bundles of the musculoskeletal system. [3]
Phase 3b: Permeating the Blood-Brain Barrier
In certain cases, a low dose of mannitol is delivered intravenously prior to the stem cell infusion. Mannitol is a sugar alcohol that temporarily opens up the blood-brain barrier so that a significantly higher percentage of cells can safely migrate into the brain. [4]
Phase 3c: IV Infusion
Because adult stem cells “home” to sites of inflammation, chronic conditions that involve low-level chronic systemic inflammation can be safely addressed with intravenous (IV) administration. An infusion of ADRCs in this manner is intended to address the the inflammation and resultant degeneration underlying many diseases of aging and reinforce the process of repair. The ADRC preparation is diluted in 100 CCs of Ringers Lactate (RL). RL is PH neutral and so preserves the viability of the diluted cells. The final cell prep is released through the vein in an arm over a 20 minute period. Patients can rest comfortably in a recovery room while this final step takes place.
Aftercare and Follow-up
Following a period of observation and recovery, patients return home to rest with a caregiver or family member. In the weeks and months that follow, the AMBROSE cycle of care continues with regular follow-up and health coaching geared towards helping our patients optimize lifestyle habits that support maximum benefit from cell therapy.
[1] M Tobita et al Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: stem cell transplantation methods that enhance stemness Stem Cell Research & Therapy (2015) 6:215
[2] V Cervelli, P Gentile, B De Angelis, et al Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post–traumatic lower extremity ulcers. StemCells 20116:103–11.
[3] A Caplan et al ADIPOSE MESENCHYMAL STEM CELLS AND “REGENERATIVE ADIPOSE TISSUE GRAFT” (LIPOGEMS™) FOR MUSCULOSKELETAL REGENERATION European Journal of Musculoskeletal Diseases Vol 2, No 2, 0-0 (2014)
[4] C.V. Borlongan et al Permeating the Blood Brain Barrier and Abrogating the Inflammation in Stroke: Implications for Stroke Therapy Curr Pharm Des. 2012; 18(25): 3670–3676
