AMBROSE Cell Therapy for Neuropathy
Pain management has long been a focus in medicine, its history dating back to ancient times. Early Egyptian, Chinese and Persian physicians first considered pain as purely a manifestation of emotions rather than the physical senses. Different theories on the expression and perception of pain have evolved through the years, but it wasn’t until 1924 that the term “neuropathy” was first used to describe a disease of the nerves. Today, it is estimated that more than 20 million people in the US have some form of neuropathy.
Research in the past decade has demonstrated that adipose-derived stem and regenerative cells (ADRCs) are effective at promoting nerve repair and thus have the potential to help improve the symptoms, function and quality of life of patients who have nerve damage in their arms and hands, legs, feet, and skin. [1] [2] [3]
The scientists investigating ADRCs have reported that this mixed population of cells reduce inflammation, rebalance the immune system, restore blood flow and regenerate tissues, nerves and organs. Through multiple biologic activities, these cells have been used safely and effectively in a wide range of conditions including the treatment of neuropathy. ADRCs are akin to having a personalized biologic “fire, rescue and repair crew” that naturally resides in our bodies. They wait quietly for a sign of trouble—inflammation—and then home to that site to do their jobs. [4] [5] [6]
Nerve Damage
Neuropathic pain arises from damage to the nervous system itself. It’s often stabbing, electrical, or burning, but any quality of pain is possible. Unfortunately, it’s also more likely to lead to chronic pain: nerves don’t heal well on their own. System-wide inflammation is a key player in the disease process of neuropathies.[7] The effects of neuropathy depend on the type of nerves they affect. Many neuropathies affect all three kinds of nerves.
Motor neuropathy impairs the movement of muscles under conscious control, such as those used for walking, grasping things, or talking.
Sensory neuropathy changes how we feel light touch, temperature, body position, or the pain from a cut.
Autonomic neuropathy interrupts the automatic control of organs to regulate things like digesting food, breathing, and heart and gland functions.
Causes of Neuropathy
Physical injury is the most common cause of single nerve damage. Accidents and surgeries can stretch, crush, swell, or compress peripheral nerves.
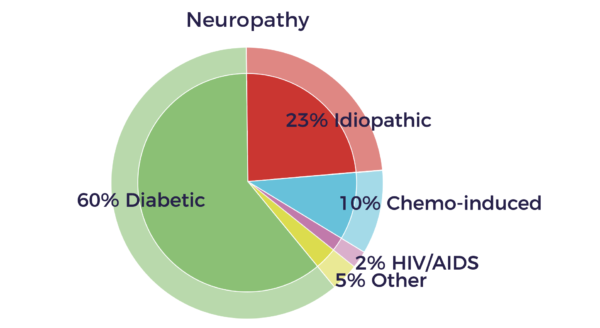
Diabetes is the leading cause of polyneuropathy, and it can affect sensory, motor, and autonomic nerves.
Poor blood flow to nerves diminishes the delivery of oxygen, and results in damaged nerves.
Systemic (body-wide) autoimmune diseases, in which the immune system mistakenly attacks a number of the body’s own tissues, can target the nerves. For example, Sjögren’s syndrome, lupus, and rheumatoid arthritis can cause neuropathic pain.
Infections and viruses such as varicella-zoster virus (which causes chicken pox and shingles), West Nile virus, cytomegalovirus, and herpes simplex target sensory fibers, causing attacks of sharp, lightning-like pain. Lyme disease, a bacterial infection transmitted by tick bites, can cause a range of neuropathic symptoms. Autoimmune diseases that attack nerves only are often triggered by infectious agents, such as seen in Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, and multifocal motor neuropathy.
Medications can cause neuropathy, particularly cancer chemotherapy drugs.
Chemical toxins can target the nervous system, such as insecticides and solvents, or heavy metals like lead, mercury, and thallium.
Unfortunately, a large number neuropathies are idiopathic, with no known cause. Several new reports now show evidence for peripheral nerve abnormalities in fibromyalgia patients that could contribute to their chronic pain.[8]
Demyelination – Remyelination
Neuropathy is a neuro-inflammatory disease in which the immune system attacks the insulation (myelin sheathing) of the nerves of the PNS, leading to demyelination.[9] Myelin sheathing is similar to the idea of coating an electrical wire with insulation to protect the metal underneath it. A nerve without myelin sheathing transmits the electrical nerve impulse very slowly, defectively, or not at all.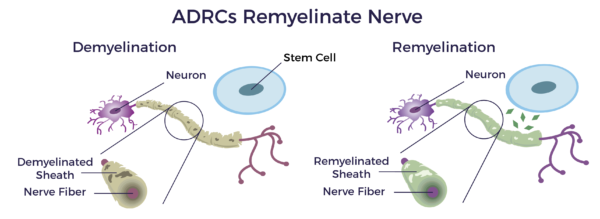
Studies have demonstrated that adipose-derived stem cells have the potential to remyelinate demyelinated nerves associated with neuropathy and other demyelinating diseases.[10] [11]
Inflammatory-Immune Response
Acute inflammation can be triggered by a trauma, infection, chemicals, environmental toxins, lifestyle choices (e.g. smoking), hereditary factors or any combination of these or other factors. This type of inflammation is essential for repairing the body, and is usually short-lived, disappearing once the healing process has taken place.
Inflammation is only problematic when it lingers, becoming chronic, and affects the entire body, becoming systemic. Chronic, systemic inflammation is often a final common pathway of neuropathy.[12] Systemic inflammation is a common factor in diseases of aging—this covers a broad spectrum of severe, debilitating and, sometimes, life-threatening conditions.[13]
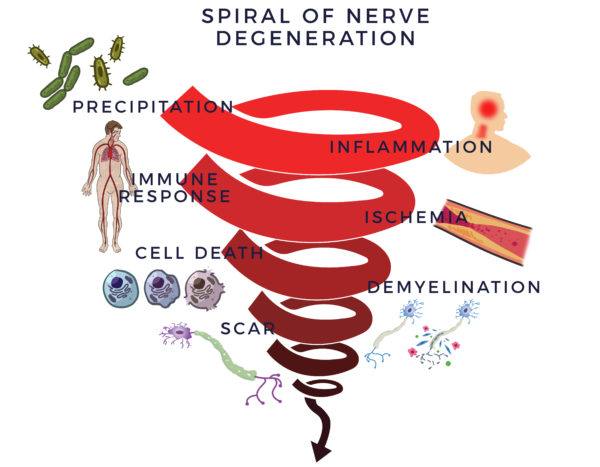
An inflammatory response begins a vicious and detrimental process. It recruits the immune system, which is there to fight infections and other disease processes, to assist healing. The cells in the immune system (immune cells) are there to guard the body. When they sense the enemy, they send out troops of molecules called “cytokines” to fight them off. When this process goes out of control, it is called an inflammatory-immune response.
The immune response is tantamount to having a backseat driver who is chronically overreacting while “helping” you drive your car. It then leads to reduced blood flow (ischemia). Without good circulation, cells die off. In neuropathy, this causes the myelin sheathing to degenerate which will then lead to scar to form in its place. We call this the Spiral of Degeneration.
Process of Repair
Through a mechanism of cell-to cell communication known as the paracrine effect, ADRCs mobilize nearby cells to work more efficiently.
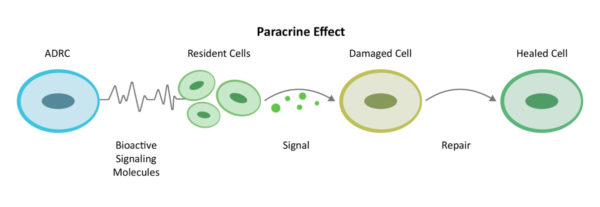
Recruiting additional “repairmen” at the site (resident stem cells) to get back on the job and do their part, ADRCs assemble an extended team and work first to decrease inflammation and the overactive immune responses. Once the backseat driving diminishes, they continue their work by increasing circulation with new blood vessel growth, preventing further cell death, decreasing scar size and finally the regeneration of healthy tissue and nerves.
This is the body’s natural healing process, sometimes it just needs reinforcements. We call this the Process of Repair.
Bioactive Molecules
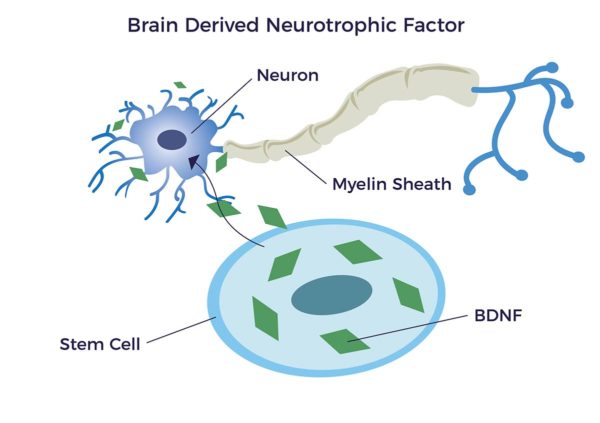
ADRCs emit hundreds of bioactive molecules that address the complex effects in the peripheral nervous system as well as other tissues and organs that are affected by or causes of neuropathy. Very relevant and important to PN are a family of biomolecules you could think of as an organic fertilizer for our nerves. These are called neurotrophic factors (NTFs). (Neuro-, relating to nerve; –trophic from Ancient Greek τροφικός [trophikós] meaning “pertaining to food or nourishment.”)
NTFs support the growth, survival, and differentiation of both developing and mature nerve cells (neurons) including new myelin sheathing. It has been shown that adipose-derived stem cells release brain-derived neurotrophic growth factor (BDNF) which promotes nerve healing and nerve growth. [14] [15] [16] [17]
AMBROSE Cell Therapy for Neuropathy
AMBROSE Cell Therapy represents a minimally invasive option to improve symptoms, function and quality of life for patients with neuropathy. The AMBROSE protocol includes a dual treatment approach for neuropathy, addressing both the deep and shallow nerves. Local nerve pain is addressed by injecting on the sides of the neurovascular bundles (perineural) under ultrasound guidance.
Please contact us for more information about treatment, candidacy and how to become a patient.
[1] T. Lopatina et al. (2011) Adipose-Derived Stem Cells Stimulate Regeneration of Peripheral Nerves: BDNF Secreted by These Cells Promotes Nerve Healing and Axon Growth De Novo. PLoS ONE 6(3): e17899
[2] JY Zhou et al Mesenchymal stem cells to treat diabetic neuropathy: a long and strenuous way from bench to the clinic Cell Death Discovery (2016) 2, e16055
[3] R Zhang, JM Rosen. The role of undifferentiated adipose-derived stem cells in peripheral nerve repair. Neural Regen Res 2018;13:757-63
[4] JK Fraser PhD and S. Kesten MD Autologous Adipose Derived Regenerative Cells: A platform for therapeutic applications Advanced Wound Healing Surgical Technology International XXIX
[5] A Nguyen, A et al Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature Journal of Plastic, Reconstructive & Aesthetic Surgery (2016) 69, 170e179
[6] Guo et al Stromal vascular fraction: A regenerative reality? Part 2: Current concepts and review of the literature Journal of Plastic, Reconstructive & Aesthetic Surgery (2016) 69, 180e188
[7] R Pop-Busui et al Inflammation as a Therapeutic Target for Diabetic Neuropathies Curr Diab Rep. 2016 March; 16(3): 29
[8] AL Oaklander et al Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. PAIN: November 2013 – Volume 154 – Issue 11 – p 2310–2316
[9] A Ellis, D.L.H. Bennett Neuroinflammation and the generation of neuropathic pain, British Journal of Anaesthesia, Volume 111, Issue 1, 2013, Pages 26-37
[10] A Hedayatpour et al. Promotion of remyelination by adipose mesenchymal stem cell transplantation in a cuprizone model of multiple sclerosis. Cell J. 2013; 15(2): 142-151
[11] N Ghasemi Therapeutic effects of adipose derived mesenchymal stem cells on remyelination process in inflammatory demyelinating diseases Journal of Histology & Histopathology 2015
[12] S. Amor Inflammation in neurodegenerative diseases Immunology, 129, 154–169
[13] C. Franceschi and J. Campisi Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age- Associated Diseases J Gerontol A Biol Sci Med Sci 2014 June;69(S1): S4–S9
[14] Razavi, Shahnaz et al. “Neurotrophic Factors and Their Effects in the Treatment of Multiple Sclerosis.” Advanced Biomedical Research 4 (2015): 53. PMC. Web. 28 Sept. 2018.
[15] J. K. Huang et al Myelin Regeneration in Multiple Sclerosis: Targeting. Endogenous Stem Cells., The American Society for Experimental NeuroTherapeutics, Inc. 2011
[16] T Lopatina et al. (2011) Adipose-Derived Stem Cells Stimulate Regeneration of Peripheral Nerves: BDNF Secreted by These Cells Promotes Nerve Healing and Axon Growth De Novo. PLoS ONE 6(3): e178991
[17] S. Seigo et al, Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration, Journal of Orthopaedic Science, Volume 18, Issue 1,2013, Pages 145-151