AMBROSE Cell Therapy for Kidney Disease
In 2010, as is typical in a new area of research, a group of scientists performed an animal study to test the potential safety and effectiveness of adipose-derived (from fat) stem and regenerative cells (ADRCs) to treat acute kidney disease.
The results were surprisingly positive, demonstrating that ADRC therapy drastically reduced mortality with 100% of the treated mice surviving as compared to 57% that received the placebo (controls). Likewise, the treated mice had significantly reduced serum creatinine levels vs. the controls.[1] Since that study, a series of other studies of acute and chronic kidney disease in small and large animals as well as humans have shown that adult stem cells and other regenerative cells from adipose tissue can potentially help to prevent disease progression, lower serum creatinine levels, reverse fibrous scar (fibrosis) and improve blood flow.[2] [3] [4] Human studies have also shown improved kidney function after stem cell treatment.[5]
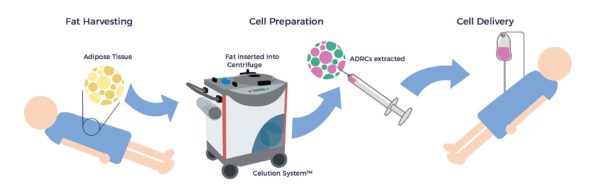
Adipose-Derived Stem and Regenerative Cells (ADRCs)
Body fat, medically known as adipose tissue, is an attractive source of stem and regenerative cells due to its accessibility, abundance and potency compared to other tissue sources such as bone marrow, umbilical cord and placenta. ADRCs can be harvested, processed and re-injected at the bedside in a same-day procedure, and through multiple biologic activities, have been used to improve symptoms, function and quality of life in a wide range of conditions, including acute and chronic kidney disease.[6] [7]
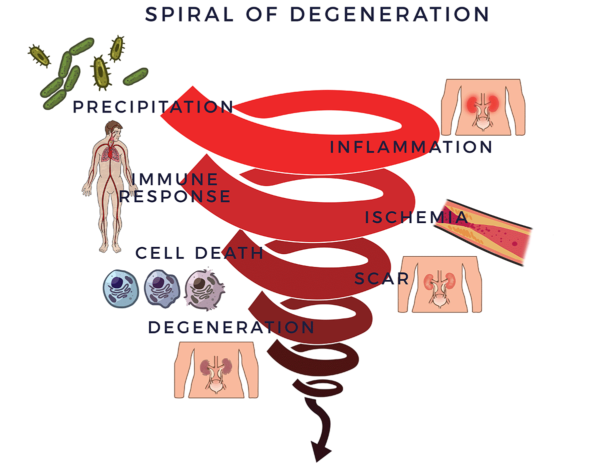
Spiral of Degeneration
Chronic degenerative conditions, including renal failure, follow a similar pattern in their disease process.
Trauma, infection, environmental toxins, unhealthy lifestyle choices (e.g. smoking), hereditary factors or a combination of these negative factors can trigger an inflammatory response. This type of inflammation (acute) is essential for repairing the body, and is usually short-lived, disappearing once the healing process has taken place.
There is another type of lingering inflammatory response which becomes chronic, and affects the entire body, becoming systemic.[8] Systemic inflammation is a common factor in diseases of aging—this covers a broad spectrum of severe, debilitating and, sometimes, life-threatening conditions, again including renal failure.[9] [10]
In the case of degenerative diseases, the chronic inflammation begins a vicious degenerative process. It recruits the immune system, which is there to fight infections and other disease processes, and to assist healing. The cells in the immune system (immune cells) are there to guard the body. When they sense the enemy, they send out troops of pro-inflammatory molecules called “cytokines” to fight them off. When this process goes out of control, it is called an inflammatory-immune response. This response is tantamount to having a backseat driver who is chronically overreacting while “helping” you drive your car. The immune response then leads to reduced blood flow (ischemia). Without good circulation, cells die off, scars and fibrosis form, tissues and organs degenerate. We call this the Spiral of Degeneration as use it as a framework for understanding some of the key factors of renal disease.
Loss of kidney function corresponds with inflammation affecting the renal (kidney) filtering system and their small blood vessels. Chronic kidney disease is well known to be associated with diabetes and uncontrolled high blood pressure, gradually worsening renal function over time. The early stages are usually without symptoms. Acute kidney disease can be rapidly provoked by an insufficient fluid status (from a variety of conditions) and from some medications.
Process of Repair
Through a mechanism of cell-to cell communication known as the paracrine effect, ADRCs mobilize nearby cells to work more efficiently. They recruit the biologic fire, rescue and repair departments at the site—resident stem cells—to get back on the job and do their part.
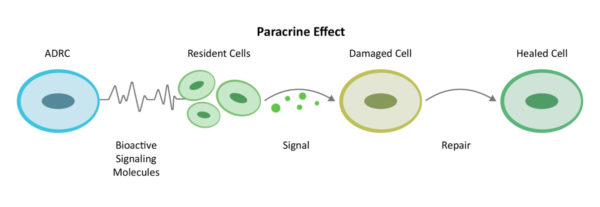
Recruiting additional “repairmen” at the site (resident stem cells) to get back on the job and do their part, ADRCs assemble an extended team and work first to decrease inflammation and the overactive immune responses. Once the backseat driving diminishes, they continue their work by increasing circulation with new blood vessel growth, preventing further cell death, addressing scar tissue and regenerating healthy tissue and nerves. This is how the body naturally heals, but if the insult from an acute or chronic condition is too great it needs help.
We call this the Process of Repair. It involves the multiple mechanisms of action that are needed to bring the systemic inflammation and immune response back to balance.
AMBROSE Cell Therapy for Kidney Disease
Because the primary factors in the Spiral discussed above are found to be involved with renal failure and the multiple repair mechanisms of the ADRCs have been shown to reverse those effects in this condition as well as a range of other serious conditions, patients with kidney disease can potentially benefit from AMBROSE Cell Therapy.
Animal studies have shown that stem cells reduce acute kidney injury by activating specialized white blood cells that initiate healing and kidney tissue repair, and protect against premature cell aging.[11] [12] [13]
Likewise, stem cell treatment of animal chronic kidney disease has been shown to promote recovery of kidney cell energy production[14] [15], decrease scarring[16], and decrease inflammation.[17]
AMBROSE Cell Therapy represents a minimally invasive treatment option for patients with renal disease. Please contact us for more information about treatment, candidacy and how to become a patient.
[1] Z Feng et al Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia–reperfusion-induced acute kidney injury, Nephrology Dialysis Transplantation, Volume 25, Issue 12, 1 December 2010, Pages 3874–3884
[2] C Donizetti-Oliveira Adipose Tissue-Derived Stem Cell Treatment Prevents Renal Disease Progression Cell Transplantation, Vol. 21, pp. 1727–1741, 2012
[3] A Eirin et al Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis Stem Cells. 2012 May; 30(5): 1030–1041
[4] JJ Rivera-Valdés et al. (2017) Human adipose derived stem cells regress fibrosis in a chronic renal fibrotic model induced by adenine. PLoS ONE 12(12)
[5] El‐Ansary M, Saadi G, Abd El‐Hamid SM. Mesenchymal stem cells are a rescue approach for recovery of deteriorating kidney function. Nephrology 2012;17:650–657.
[6] Guo et al Stromal vascular fraction: A regenerative reality? Part 2: Current concepts and review of the literature Journal of Plastic, Reconstructive & Aesthetic Surgery (2016) 69, 180e188
[7] JK Fraser PhD and S Kesten MD Autologous Adipose Derived Regenerative Cells: A platform for therapeutic applications Advanced Wound Healing Surgical Technology International XXIX
[8] S. Amor Inflammation in neurodegenerative diseases Immunology, 129, 154–169
[9] C. Franceschi and J. Campisi Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases J Gerontol A Biol Sci Med Sci 2014 June;69(S1): S4–S9
[10] Suliman ME, Stenvinkel P. Contribution of Inflammation to Vascular Disease in Chronic Kidney Disease Patients. Saudi J Kidney Dis Transpl 2008;19:329-45
[11] Geng, Y.; Zhang, L.; Fu, B.; Zhang, J.; Hong, Q.; Hu, J.; Li, D.; Luo, C.; Cui, S.; Zhu, F.; et al. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of m2 macrophages. Stem Cell Res. Ther. 2014, 5, 80
[12] Lee, S.J.; Ryu, M.O.; Seo, M.S.; Park, S.B.; Ahn, J.O.; Han, S.M.; Kang, K.S.; Bhang, D.H.; Youn, H.Y. Mesenchymal stem cells contribute to improvement of renal function in a canine kidney injury model. In Vivo 2017, 31, 1115–1124
[13] Rodrigues, C.E.; Capcha, J.M.; de Braganca, A.C.; Sanches, T.R.; Gouveia, P.Q.; de Oliveira, P.A.; Malheiros, D.M.; Volpini, R.A.; Santinho, M.A.; Santana, B.A.; et al. Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res. Ther. 2017, 8, 19
[14] Yoon, Y.M.; Han, Y.S.; Yun, C.W.; Lee, J.H.; Kim, R.; Lee, S.H. Pioglitazone protects mesenchymal stem cells against p-cresol-induced mitochondrial dysfunction via up-regulation of pink-1. Int. J. Mol. Sci. 2018, 19, 2898
[15] Lira, R.; Oliveira, M.; Martins, M.; Silva, C.; Carvalho, S.; Stumbo, A.C.; Cortez, E.; Verdoorn, K.; Einicker-Lamas, M.; Thole, A.; et al. Transplantation of bone marrow-derived mscs improves renal function and na(+)+k(+)-atpase activity in rats with renovascular hypertension. Cell Tissue Res. 2017, 369, 287–301
[16] Wu, H.J.; Yiu, W.H.; Li, R.X.; Wong, D.W.; Leung, J.C.; Chan, L.Y.; Zhang, Y.; Lian, Q.; Lin, M.; Tse, H.F.; et al. Mesenchymal stem cells modulate albumin-induced renal tubular inflammation and fibrosis. PLoS ONE 2014, 9, e90883
[17] Abdel Aziz, M.T.; Wassef, M.A.; Ahmed, H.H.; Rashed, L.; Mahfouz, S.; Aly, M.I.; Hussein, R.E.; Abdelaziz, M. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol. Metab. Syndr. 2014, 6, 34