About AMBROSE
AMBROSE Cell Therapy was founded in 2018 by Matthew (Matt) Feshbach with the Mission: To help people with chronic degenerative diseases improve symptoms, function, and quality of life using adult stem cell therapy.
To accomplish this, we access the mixed population of stem and regenerative cells in a patient’s fat (adipose tissue). This cell preparation, when clinical grade, is called adipose-derived stem and regenerative cells (ADRCs).
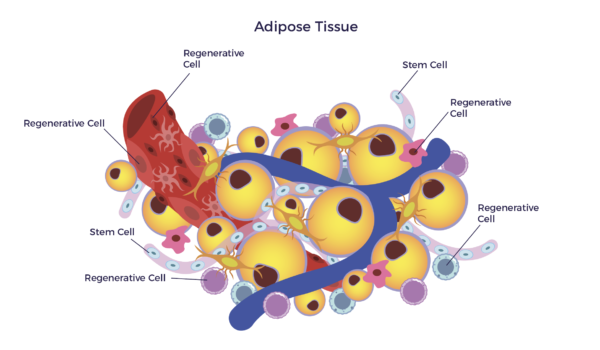
More on AMBROSE in a moment; first, a bit of the back-story:
Our journey formally began in August 2011, when our CEO, Matt, and his (deceased) brother, Joe, founded Okyanos (Oh-key-ah-nos) Cell Therapy in Freeport, Bahamas. “Okyanos,” the Greek God of Rivers, symbolizes the restoration of blood flow, which is an essential mechanism of action of ADRCs. Due to the uncompromising standard of care for their patients, Okyanos became known by many as the first purpose-built, cell therapy center of excellence in the world. Matt left Okyanos in April of 2017, but bringing with him his purpose to continue helping patients with debilitating conditions live better lives.
On May 30, 2018, the Federal Right to Try Act was signed into law. The purpose of the Right to Try Act is to empower patients who are terminally ill or have life-threatening diseases to take control of their health by having the right and opportunity to seek treatment that is not approved by the FDA.

“Right to Try” patients live with vexing complexities. Sadly, for many of these individuals, surgeries, drugs, and devices have not significantly improved their quality of life – and, often, it may be worsening. In medicine, these patients are referred to as “no-option.”
Fortuitously, the requirements of the Right to Try Act dovetailed with the technologies, protocols, and patient criteria that Matt believed would best address the unmet need of no-option patients. And so, Matt established AMBROSE Cell Therapy in August 2018.
Consistent with our history and Mission, we set the objective to provide AMBROSE Cell Therapy in a center of excellence setting. We see that as the standard that is required to help solve the wide-reaching unmet need of these patients. To that end, board-certified specialist physicians provide our cell therapy services.
Further, AMBROSE uses the Celution® cell processing system to liberate clinical-grade ADRCs. After processing, the ADRCs are then reintroduced back into the patient at the point of care.
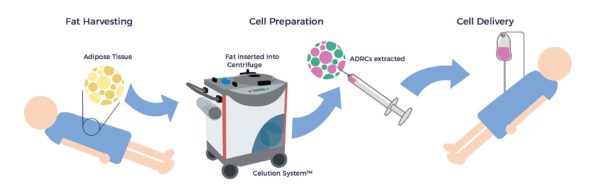
From a safety perspective, it is important for patients to know that Celution is approved in over 40 countries internationally and, has been utilized in 9 FDA-approved Phase 1/2 safety trials. Add to that, researchers from around the globe have published more than 30 human clinical studies or case reports in which Celution-processed ADRCs were used to address a broad spectrum of chronic conditions.[1] In the 12 years since Celution was approved in Europe and Japan, there has not been a single report of a cell-related adverse event.
Your personalized protocol also may involve direct injections of micronized adipose tissue. The tissue contains ADRCs resident within it. After being introduced into inflamed sites, they have been documented to release and become biologically active. Micronized fat is best used to address localized conditions involving bones, muscles, nerves, wounds, and scars.[2] [3] (For more information see The Skinny on Fat and Protocol Overview.)
As we look to the future, we are driven by our overarching purpose to help people with complex chronic diseases live better lives. Please contact us to learn more.
[1] JK Fraser PhD and S. Kesten MD Autologous Adipose Derived Regenerative Cells: A platform for therapeutic applications Advanced Wound Healing Surgical Technology International XXIX
[2] H Riyat et al Autologous fat grafting for scars, healing, and pain: a review Scars, Burns & Healing Volume 3: 1–16
[3] C Tremolada et al. Adipose Mesenchymal Stem Cells and Regenerative Adipose Tissue Graft (LipogemsÔ) for Musculoskeletal Regeneration European Journal of Musculoskeletal Diseases Vol. 3, no. 2, 0-0 (2014) 57
