Welcome to Ambrose Cell Therapy
Ambrose exists for just one purpose: To help people living with life-threatening and debilitating conditions improve their quality of life. To accomplish that, we provide patients the Right-to-Try their own (autologous) adipose-derived stem and regenerative cells (ADRCs).
“I know it sounds too good to be true – but it is true,” shared Tim. Eight months after Ambrose Cell Therapy, Tim went for a physical. “My blood pressure, blood sugar, cholesterol, REM sleep, and mood haven’t been this good since I was in my 20’s.” Other than some physical therapy to help his knee regain full function, Tim let his ADRCs do their job.
In contrast to the US and offshore stem cell clinics that incentivize celebrities and pro athletes to promote their treatments on Instagram, TikTok, YouTube, and podcasts, Ambrose’s website provides educational content to assist patients with making an informed choice.
We curate the information for our articles from:
- A broad, deep, and growing body of scientific literature, including over 125,000 papers on adipose-derived stem cells.
- Over 80 Celution-process ADRC studies.
- Ambrose’s Patient-Reported Outcomes and follow-up data.
Self-proclaimed stem cell gurus claiming to do cutting-edge treatments in Dubai, Mexico, Costa Rica, or Boca Raton don’t cut it when it comes to the 100 million patients in the US living with chronic diseases despite maximum medical management.
These patients need and are looking for a science-backed standard of care. As important, they deserve empathy, compassion, and communication. Bringing these factors to bear requires a purpose-driven team.
“From the physicians to the nurses and the administrative staff, every Ambrose team member was so impressive. The Ambrose team manifests vitality, purpose, and efficiency. You are one of the top groups of people I have ever come across in my 80 years of living. Thank you, Ambrose!” ~ ID
In summary, no-option patients have the right to be happier and healthier by accessing their ADRCs.
The best is yet to come,
Matthew (Matt) Feshbach
CEO
Ambrose Cell Therapy
Welcome to Ambrose Cell Therapy
Ambrose exists for just one purpose: To help people living with life-threatening and debilitating conditions improve their quality of life. To accomplish that, we provide patients the Right-to-Try their own (autologous) adipose-derived stem and regenerative cells (ADRCs).
“I know it sounds too good to be true – but it is true,” shared Tim. Eight months after Ambrose Cell Therapy, Tim went for a physical. “My blood pressure, blood sugar, cholesterol, REM sleep, and mood haven’t been this good since I was in my 20’s.” Other than some physical therapy to help his knee regain full function, Tim let his ADRCs do their job.
In contrast to the US and offshore stem cell clinics that incentivize celebrities and pro athletes to promote their treatments on Instagram, TikTok, YouTube, and podcasts, Ambrose’s website provides educational content to assist patients with making an informed choice.
We curate the information for our articles from:
- A broad, deep, and growing body of scientific literature, including over 125,000 papers on adipose-derived stem cells.
- Over 80 Celution-process ADRC studies.
- Ambrose’s Patient-Reported Outcomes and follow-up data.
Self-proclaimed stem cell gurus claiming to do cutting-edge treatments in Dubai, Mexico, Costa Rica, or Boca Raton don’t cut it when it comes to the 100 million patients in the US living with chronic diseases despite maximum medical management.
These patients need and are looking for a science-backed standard of care. As important, they deserve empathy, compassion, and communication. Bringing these factors to bear requires a purpose-driven team.
“From the physicians to the nurses and the administrative staff, every Ambrose team member was so impressive. The Ambrose team manifests vitality, purpose, and efficiency. You are one of the top groups of people I have ever come across in my 80 years of living. Thank you, Ambrose!” ~ ID
In summary, no-option patients have the right to be happier and healthier by accessing their ADRCs.
The best is yet to come,
Matthew (Matt) Feshbach
CEO
Ambrose Cell Therapy
Matthew (Matt) Feshbach
 . . . founded Ambrose Cell Therapy in 2018. Under the Federal Right to Try Act of 2017, Ambrose treats patients who have life-threatening and debilitating conditions with their own adipose-derived regenerative cells (ADRCs).
. . . founded Ambrose Cell Therapy in 2018. Under the Federal Right to Try Act of 2017, Ambrose treats patients who have life-threatening and debilitating conditions with their own adipose-derived regenerative cells (ADRCs).
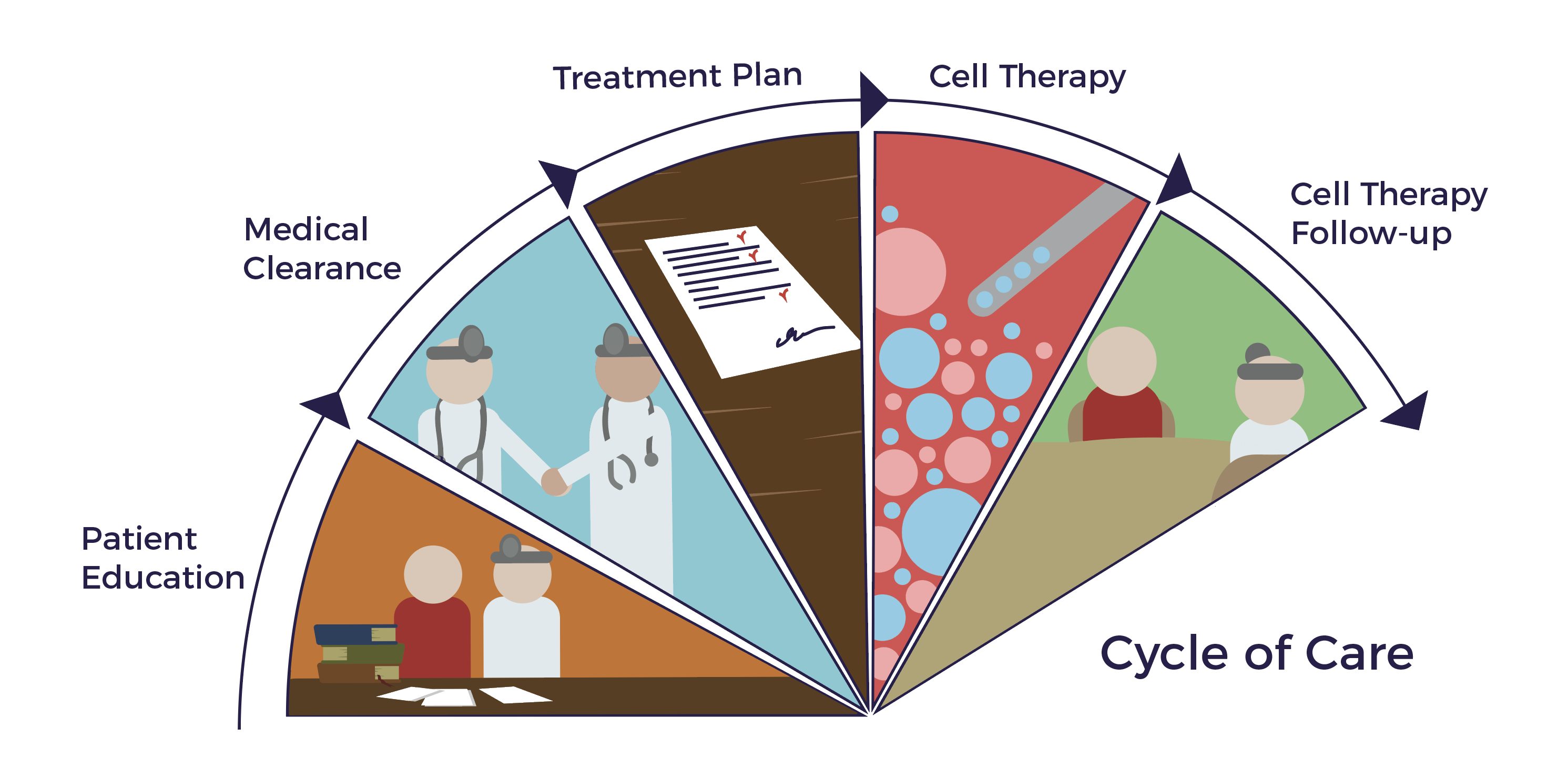
Ambrose personalizes each patient’s cell therapy plan and cycle of care based on their needs and goals.
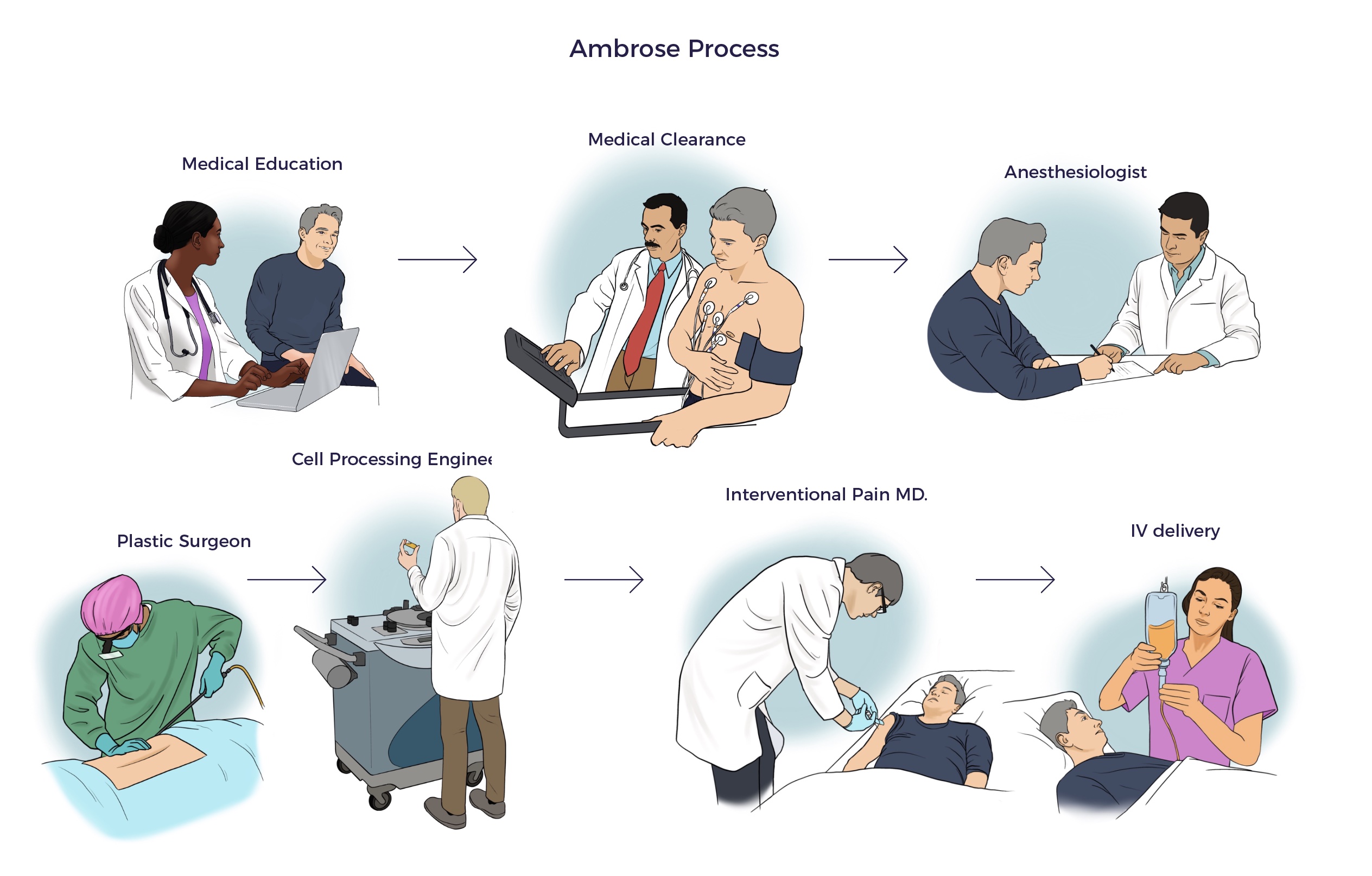
Ambrose Cell Therapy involves five doctors, including an MD for patient education, a plastic surgeon for fat harvesting, an interventional pain doctor for regenerative injections, our medical director for patient clearance, a board-certified anesthesiologist, a cell processing engineer, a director of nursing and OR staff.
The protocol includes fat harvesting, ADRC preparation, and a personalized treatment plan. Our physicians deliver Ambrose Cell Therapy in an AAAAHC- accredited surgery center.

Empowering Patients: Federal Right to Try Act
 On May 30, 2018, the Federal Right to Try Act was signed into law. The purpose of the Right to Try Act is to empower patients who are terminally ill or have life-threatening diseases to take control of their health by having the right and opportunity to seek treatment that is not approved by the FDA.
On May 30, 2018, the Federal Right to Try Act was signed into law. The purpose of the Right to Try Act is to empower patients who are terminally ill or have life-threatening diseases to take control of their health by having the right and opportunity to seek treatment that is not approved by the FDA.
“Right to Try” patients live with vexing complexities. Sadly, for many of these individuals, surgeries, drugs, and devices have not significantly improved their quality of life – and, often, it may be worsening. In medicine, these patients are referred to as “no-option.”
Fortuitously, the Right to Try Act’s requirements dovetailed with the technologies, protocols, and no-option patient criteria Matt pioneered with his prior group, Okyanos Cell Therapy, in the Bahamas. Therefore, Matt established Ambrose Cell Therapy in the US.
Ambrose Protocol
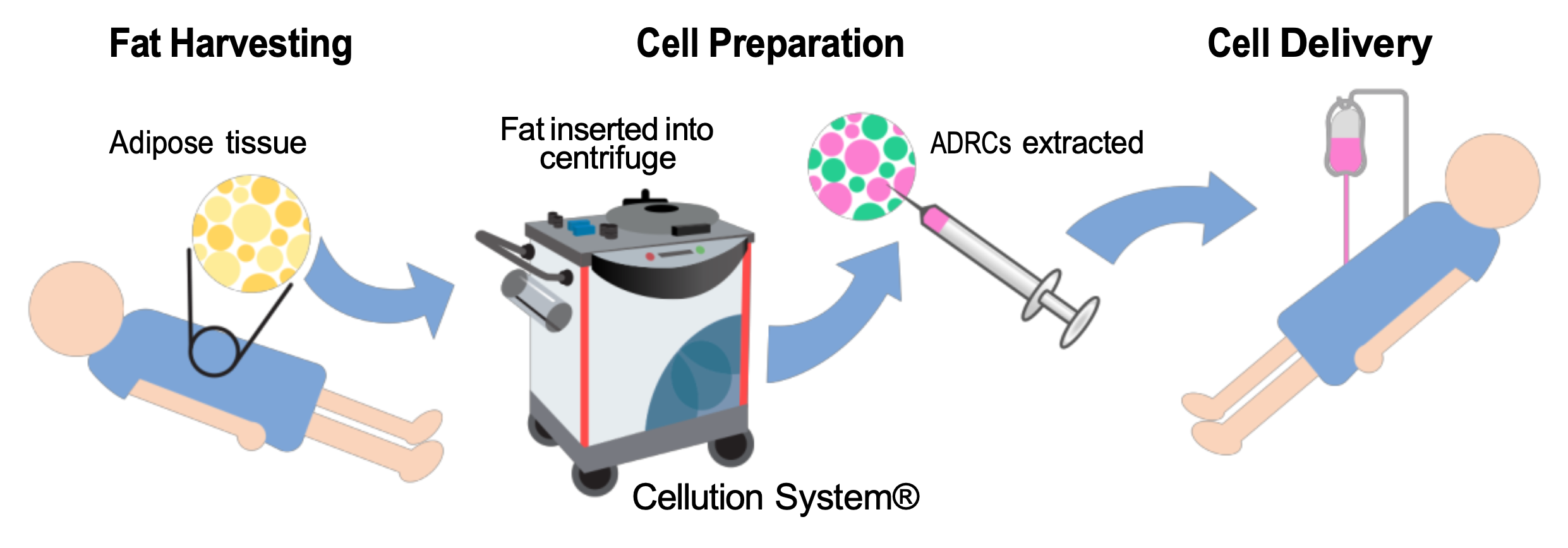
At Ambrose, we access the mixed population of stem and regenerative cells in a patient’s fat (adipose tissue). We refer to this clinical-grade cell population as adipose-derived stem and regenerative cells (ADRCs).

ADRC Processing
Ambrose uses the Celution System® (Lorem Cytori, San Diego, CA) to liberate a patient’s ADRCs from their fat. Celution is a closed, sterile system. Published studies confirm that the Celution-processed output contains the highest ADRC yield, viability, and purity of any adipose cell processing device on the market.
 Leading academic research institutions, including Cedar-Sinai, Texas Heart Institute, and Stanford University, have utilized the Celution System in nine FDA-approved trials.
Leading academic research institutions, including Cedar-Sinai, Texas Heart Institute, and Stanford University, have utilized the Celution System in nine FDA-approved trials.
In addition, researchers have published over 80 human clinical trials, case reports, or reviews.
Regulators in over 40 countries, including Japan, the EU, Australia, and China, have approved Celution for patient treatments, further validating ADRCs’ safety and effectiveness.
Ambrose Physicians and Facility
Ambrose’s objective is to be a cell therapy center of excellence. To that end, board-certified specialist physicians provide our cell therapy services in a AAAAHC-accredited surgery center.

Dr. Ram Dandillaya • MEDICAL DIRECTOR
Dr. Ram Dandillaya is the Clinical Chief in the Division of Cardiology at the Cedars-Sinai Medical Center. Dr. Ram clears or reviews PCP clearances for Ambrose patients.

Dr. Nitesh Patel • ATTENDING PHYSICIAN AND REGENERATIVE INJECTION SPECIALIST
Dr. Patel is a board-certified anesthesiologist and completed an interventional pain management fellowship at the prestigious Stanford University Medical Center.

Dr. Walter Joseph • PLASTIC SURGEON
Dr. Joseph completed his residency at the renowned University of Pittsburgh Medical Center.

Dr. Cheryl Jones • PATIENT EDUCATOR
Dr. Jones has 25 years of family medicine experience. She educates patients on Ambrose Cell Therapy to assist them in making an informed choice and obtains a brief medical history to develop a preliminary treatment plan for the patient’s consideration.

Navneet Chagger • CELL PROCESSING ENGINEER
Mr. Chaggar earned his degree in bioengineering from the University of California San Diego. He has managed the cell processing for Celution system clinical trials.
Ambrose Physician Services provides treatments at Salus ASC (ambulatory surgery center), an AAAAHC-accredited surgery center located at 50 N. La Cienega Blvd, Suite 201, Beverly Hills, CA 90211

Ambrose Patient Reported Outcomes
“I got my daily life back, and also one of my most favorite things to do was possible again. I could dream again. As I look back, my improved quality of life is amazing compared to where I started.” – AC • N EUROPATHY, FIBROMYALGIA, DIABETES
“The stem cells have already cured or greatly improved major pain in my neck and shoulder/arm pain from pinched nerves I’ve had for 20 years. Also, a sensation in my legs that felt like a thousand pin pricks. It made me crazy. Haven’t had it since the procedure THANK GOD!!! Doing better every day.” – LR • PSORIATIC ARTHRITIS
“I am in NYC celebrating my big 90. Look great, doing well and feel great. All due to stem cells I am sure.” –NK • RHEUMATOID ARTHRITIS, MINI STROKES
“After my stem cell treatment, I feel sharper and have better energy. I feel great and am doing more than I have ever done.” –MG • INJURY, CHRONIC PAIN, CONCUSSION
“The resurgence of my energy level has been amazing. It continues two years post-treatment – and now after my kidney transplant.” –KT • END STAGE KIDNEY DISEASE, ARTHRITIS
“I feel wonderful. Everything I came to be treated for [brain fog, fatigue, memory, chronic pain] is great now. I got everything I wanted out of it.” –BW • POLYMYALGIA RHEUMATICA, LONG COVID
“No one really knows how bad things were for me (except my doctor). Just before stem cell treatment I was searching different neurological care for tremors, brain fog, and emotional regulation. I had an intervention for depression and mandatory counseling for 5 weeks because I was suicidal. I was desperate. I don’t feel ‘foggy’ anymore. The cloud of depression and anxiety was gone immediately. Isn’t it amazing?” –SG • LONG COVID
