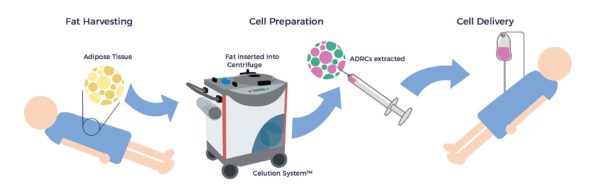
العلاج بالخلايا AMBROSE لعلاج أمراض الكلى
في عام 2010، وكما هو معتاد في مجال بحثي جديد، أجرى مجموعة من العلماء دراسة على الحيوانات لاختبار السلامة والفعالية المحتملة للخلايا الجذعية والتجديدية المشتقة من الدهون (من الدهون) لعلاج أمراض الكلى الحادة.
كانت النتائج إيجابية بشكل مدهش، حيث أظهرت أن العلاج بـ ADRC قلل بشكل كبير من معدل الوفيات حيث نجت 1001 من الفئران المعالجة مقارنة بـ 571 من الفئران التي تلقت العلاج الوهمي (الضوابط). وبالمثل، انخفضت مستويات الكرياتينين في المصل لدى الفئران المعالجة بشكل كبير مقارنةً بالفئران التي تلقت العلاج الوهمي (الضوابط).[1] منذ تلك الدراسة، أظهرت سلسلة من الدراسات الأخرى التي أجريت على أمراض الكلى الحادة والمزمنة في الحيوانات الصغيرة والكبيرة وكذلك البشر أن الخلايا الجذعية البالغة وغيرها من الخلايا التجددية من الأنسجة الدهنية يمكن أن تساعد في منع تطور المرض، وخفض مستويات الكرياتينين في الدم، وعكس الندبة الليفية (التليف) وتحسين تدفق الدم.[2] [3] [4] كما أظهرت الدراسات التي أُجريت على البشر تحسنًا في وظائف الكلى بعد العلاج بالخلايا الجذعية.[5]
الخلايا الجذعية والتجديدية المشتقة من الدهون (ADRCs)
تُعد دهون الجسم، والمعروفة طبياً باسم الأنسجة الدهنية، مصدراً جذاباً للخلايا الجذعية والتجديدية نظراً لسهولة الوصول إليها ووفرتها وفعاليتها مقارنة بمصادر الأنسجة الأخرى مثل نخاع العظم والحبل السري والمشيمة. يمكن حصاد الخلايا الجذعية الجذعية الدهنية الجذعية ومعالجتها وإعادة حقنها في نفس اليوم في إجراء جراحي في نفس اليوم، ومن خلال أنشطة بيولوجية متعددة، تم استخدامها لتحسين الأعراض والوظائف ونوعية الحياة في مجموعة واسعة من الحالات، بما في ذلك أمراض الكلى الحادة والمزمنة.[6] [7]
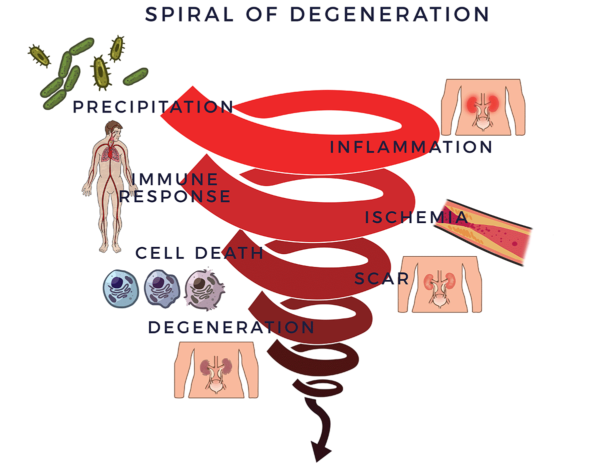
دوامة الانحطاط
وتتبع الحالات التنكسية المزمنة، بما في ذلك الفشل الكلوي، نمطاً مماثلاً في عملية المرض.
يمكن أن تؤدي الصدمة أو العدوى أو السموم البيئية أو خيارات نمط الحياة غير الصحية (مثل التدخين) أو العوامل الوراثية أو مزيج من هذه العوامل السلبية إلى حدوث استجابة التهابية. هذا النوع من الالتهاب (الحاد) ضروري لإصلاح الجسم، وعادةً ما يكون قصير الأجل، ويختفي بمجرد حدوث عملية الشفاء.
هناك نوع آخر من الاستجابة الالتهابية المستمرة التي تصبح مزمنة، وتؤثر على الجسم بأكمله، وتصبح جهازية.[8] يعد الالتهاب الجهازي عاملاً شائعاً في أمراض الشيخوخة - وهذا يغطي طيفاً واسعاً من الحالات المرضية الشديدة والموهنة وأحياناً المهددة للحياة، بما في ذلك الفشل الكلوي أيضاً.[9] [10]
في حالة الأمراض التنكسية، يبدأ الالتهاب المزمن في عملية تنكسية شرسة. حيث يقوم بتجنيد الجهاز المناعي الموجود لمحاربة الالتهابات وعمليات المرض الأخرى والمساعدة في الشفاء. توجد خلايا الجهاز المناعي (الخلايا المناعية) لحراسة الجسم. وعندما تشعر بالعدو، فإنها ترسل قوات من الجزيئات المؤيدة للالتهابات التي تُسمى “السيتوكينات” لمكافحته. وعندما تخرج هذه العملية عن نطاق السيطرة، يُطلق عليها الاستجابة المناعية الالتهابية. هذه الاستجابة هي بمثابة وجود سائق في المقعد الخلفي يبالغ في رد فعله بشكل مزمن بينما “يساعدك” في قيادة سيارتك. تؤدي الاستجابة المناعية بعد ذلك إلى انخفاض تدفق الدم (نقص التروية). وبدون دورة دموية جيدة، تموت الخلايا وتتشكل الندوب والتليف وتتدهور الأنسجة والأعضاء. نطلق على هذه الحالة اسم "دوامة التنكس" حيث نستخدمها كإطار لفهم بعض العوامل الرئيسية لأمراض الكلى.
يتوافق فقدان وظائف الكلى مع الالتهابات التي تؤثر على نظام الترشيح الكلوي (الكلى) وأوعيتها الدموية الصغيرة. من المعروف أن مرض الكلى المزمن يرتبط بمرض السكري وارتفاع ضغط الدم غير المنضبط، مما يؤدي إلى تدهور وظائف الكلى تدريجياً مع مرور الوقت. عادةً ما تكون المراحل المبكرة بدون أعراض. يمكن أن يحدث مرض الكلى الحاد بسرعة بسبب نقص السوائل (من مجموعة متنوعة من الحالات) ومن بعض الأدوية.
عملية الإصلاح
من خلال آلية التواصل بين الخلايا المعروفة باسم تأثير الباراكرين، تحشد الخلايا الجذعية المقيمة في الموقع لتعمل بكفاءة أكبر. فهي تقوم بتجنيد أقسام الإطفاء والإنقاذ والإصلاح البيولوجية في الموقع - الخلايا الجذعية المقيمة - لتعود إلى العمل والقيام بدورها.
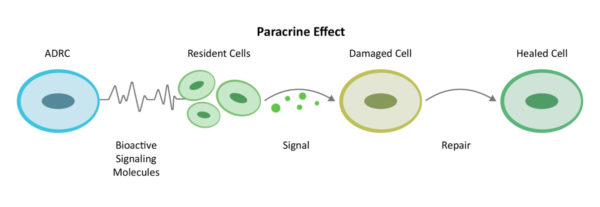
من خلال تجنيد "مصلحين" إضافيين في الموقع (الخلايا الجذعية المقيمة) للعودة إلى العمل والقيام بدورهم، تقوم الخلايا الجذعية المقيمة بتجميع فريق موسع وتعمل أولاً على تقليل الالتهاب والاستجابات المناعية المفرطة النشاط. وبمجرد أن تتضاءل القيادة الخلفية، تواصل الخلايا الجذعية المقيمة عملها من خلال زيادة الدورة الدموية مع نمو أوعية دموية جديدة، ومنع المزيد من موت الخلايا، ومعالجة النسيج الندبي وتجديد الأنسجة والأعصاب السليمة. هذه هي الطريقة التي يتعافى بها الجسم بشكل طبيعي، ولكن إذا كانت الإهانة الناجمة عن حالة حادة أو مزمنة كبيرة للغاية فإنها تحتاج إلى المساعدة.
نسمي هذه العملية بعملية الإصلاح. وهي تتضمن آليات العمل المتعددة اللازمة لإعادة الالتهاب الجهازي والاستجابة المناعية إلى التوازن.
العلاج بالخلايا AMBROSE لعلاج أمراض الكلى
ونظراً لأن العوامل الأساسية في الحلزونية التي تمت مناقشتها أعلاه قد وُجد أنها مرتبطة بالفشل الكلوي وثبت أن آليات الإصلاح المتعددة التي تستخدمها خلايا أمبروز الحلزونية قد أثبتت قدرتها على عكس هذه التأثيرات في هذه الحالة بالإضافة إلى مجموعة من الحالات الخطيرة الأخرى، فإن المرضى الذين يعانون من أمراض الكلى يمكن أن يستفيدوا من العلاج بالخلايا من أمبروز.
أظهرت الدراسات التي أُجريت على الحيوانات أن الخلايا الجذعية تقلل من إصابة الكلى الحادة عن طريق تنشيط خلايا الدم البيضاء المتخصصة التي تبدأ في الشفاء وإصلاح أنسجة الكلى، وتحمي من شيخوخة الخلايا المبكرة.[11] [12] [13]
وبالمثل، فقد ثبت أن علاج الخلايا الجذعية لأمراض الكلى المزمنة لدى الحيوانات يعزز من تعافي إنتاج خلايا الكلى للطاقة[14] [15], تقليل التندب[16], وتقليل الالتهاب.[17]
يمثل العلاج بالخلايا AMBROSE خياراً علاجياً طفيف التوغل للمرضى الذين يعانون من أمراض الكلى. يُرجى الاتصال بنا للحصول على مزيد من المعلومات حول العلاج والترشح وكيفية الحصول على العلاج.
[1] زد فنغ وآخرون خلايا جذعية وتجديدية طازجة ومحفوظة بالتبريد وغير مزروعة من الأنسجة الدهنية المستمدة من الأنسجة الدهنية تخفف من إصابة الكلى الحادة الناجمة عن نقص التروية وإعادة ضخ الدم, غسيل الكلى وزراعة الكلى وزراعة الكلى، المجلد 25، العدد 12، 1 ديسمبر 2010، الصفحات 3874-3884
[2] سي دونيزيتي-أوليفيرا علاج الخلايا الجذعية المشتقة من الأنسجة الدهنية يمنع تطور مرض الكلى زرع الخلايا، المجلد 21، ص 1727-1741، 2012
[3] A Eirin et al الخلايا الجذعية الوسيطة الوسيطة المشتقة من الأنسجة الدهنية تحسن نتائج إعادة التوعية، لاستعادة وظيفة الكلى في الخنازير في حالة تصلب الشرايين الكلوية تضيق الشريان الكلوي الخلايا الجذعية. 2012 مايو؛ 30(5): 1030-1041
[4] JJ Rivera-Valdés et al. (2017) الخلايا الجذعية البشرية المشتقة من الخلايا الجذعية الدهنية المشتقة من الدهون البشرية تعمل على إصلاح التليف في نموذج التليف الكلوي المزمن الناجم عن الأدينين. PLoS ONE 12 (12)
[5] الأنصاري م، سعدي ج، عبد الحميد م. الخلايا الجذعية الوسيطة الوسيطة هي نهج إنقاذ لاستعادة وظائف الكلى المتدهورة. أمراض الكلى 2012;17:650-657.
[6] قوه وآخرون جزء الأوعية الدموية اللحمية: واقع متجدد؟ الجزء 2: المفاهيم الحالية ومراجعة الأدبيات مجلة الجراحة التجميلية والترميمية والتجميلية (2016) 69, 180e188
[7] دكتوراه في الطب JK Fraser و S Kesten MD الخلايا التجددية الذاتية المشتقة من الدهون: منصة للتطبيقات العلاجية التكنولوجيا الجراحية الدولية المتقدمة لالتئام الجروح الجراحية التاسعة والعشرون
[8] S. Amor Inflammation in neurodegenerative diseases Immunology, 129, 154-169
[9] C. Franceschi and J. Campisi الالتهاب المزمن (الالتهاب) ومساهمته المحتملة في الأمراض المرتبطة بالعمر J Gerontol A Biol Sci Med Sci 2014 June;69(S1): S4-S9
[10] Suliman ME, Stenvinkel P. مساهمة الالتهاب في أمراض الأوعية الدموية لدى مرضى أمراض الكلى المزمنة. Saudi J Kidney Dis Transpl 2008; 19:329-45
[11] Geng, Y.; Zhang, L.; Fu, B.; Zhang, J.; Hong, Q.; Hu, J.; Li, D.; Luo, C.; Cui, S.; Zhu, F.; et al. الخلايا الجذعية الوسيطة اللُّحمية الوسيطة تخفف من إصابة الكلى الحادة الناجمة عن انحلال الربيدات عن طريق تنشيط الخلايا البلعمية الكبيرة m2. Stem Cell Res. Ther. 2014, 5, 80
[12] Lee, S.J.; Ryu, M.O.; Seo, M.S.; Park, S.B.; Ahn, J.O.; Han, S.M.; Kang, K.S.; Bhang, D.H.; Youn, H.Y. تساهم الخلايا الجذعية الوسيطة في تحسين وظيفة الكلى في نموذج إصابة الكلى في الكلاب. في الجسم الحي 2017، 31، 31، 1115-1124
[13] Rodrigues, C.E.; Capcha, J.M.; de Braganca, A.C.; Sanches, T.R.; Gouveia, P.Q.; de Oliveira, P.A.; Malheiros, D.M.; Volpini, R.A.; Santinho, M.A.; Santana, B.A.; et al. الخلايا اللحمية الوسيطة اللحمية الوسيطة المشتقة من الحبل السري البشري تحمي من الشيخوخة الكلوية المبكرة الناتجة عن الإجهاد التأكسدي في الفئران المصابة بإصابة حادة في الكلى. Stem Cell Res. Ther. 2017, 8, 19
[14] Yoon, Y.M.; Han, Y.S.; Yun, C.W.; Lee, J.H.; Kim, R.; Lee, S.H. Pioglitazone يحمي الخلايا الجذعية الوسيطة من الخلل الوظيفي للميتوكوندريا الناجم عن الكريسول p- كريسول عبر التنظيم الأعلى للوردي-1. Int. J. Mol. Sci. 2018, 19, 2898
[15] Lira, R.; Oliveira, M.; Martins, M.; Silva, C.; Carvalho, S.; Stumbo, A.C.; Cortez, E.; Verdoorn, K.; E.; E. Verdoorn, K.; Einicker-Lamas, M.; Thole, A.; et al. زرع النخاع العظمي المشتق من نخاع العظم يحسن وظيفة الكلى ونشاط na(+) +k(+) -atpase في الفئران المصابة بارتفاع ضغط الدم الوعائي الجديد. Cell Tissue Res. 2017, 369, 287-301
[16] Wu, H.J.; Yiu, W.H.; Li, R.X.; Wong, D.W.; Leung, J.C.; Chan, L.Y.; Zhang, Y.; Lian, Q.; Lin, M.; Tse, H.F.; et al. Mesenchymal stem cells modulate-induireced Albumin-induced الكلوي الأنبوبي والتليف. PLoS ONE 2014, 9, e90883
[17] عبد العزيز، م. ت.؛ واصف، م. أ.؛ أحمد، ح. ح.؛ راشد، ل.؛ محفوظ، س.؛ علي، م. إ.؛ حسين، ر. إ.؛ عبد العزيز، م. دور الخلايا الجذعية المشتقة من نخاع العظم-المتوسطة في توهين وظائف الكلى في الفئران المصابة باعتلال الكلية السكري. Diabetol. ميتاب. Syndr. 2014, 6, 34